Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose:
EGPA is a small vessel vasculitis characterized by the presence of tissue eosinophilia, necrotizing vasculitis and granulomatous inflammation1. In the randomized, placebo-controlled MIRRA trial for relapsing and refractory EGPA, adjuvant therapy with 300mg anti-IL5 mAB Mepolizumab [MEPO], accrued longer times in remission, reduced steroid exposure and reduced relapse rates2. The aim of the study was to analyze the longer term outcome for EGPA patients who received MEPO monthly for 18 months [M] and beyond, at 100mg dosage.
Methods: This retrospective, descriptive study analyzed 13 patients with EGPA, who received 100mg s/c of MEPO therapy monthly. Time points of assessment included MEPO commencement [M0], month 12 [M12] and ≧ month 18 [M ≧ 18].
Results: This study demonstrates that anti-IL5 therapy serves as a favorable model for steroid minimization in EGPA, with an overall 50% reduction in steroid dosage. Adjuvant conventional immunosuppressants were stopped in 3 patients, and commenced in two. By 12 months, ANCA serology normalized in all four positive patients, BVAS reduced [n=13, mean±SD, 7.3±6.2 M0 to 2.23 ±1.69 M12], along with reduction in asthma Control questionnaire [n=5, mean ± SD, 2.92 ±1.27 M0 to 2.23±1.69 M12]. Well tolerated, at M≥18, anti-IL5 therapy demonstrated considerable clinical benefit, with 12 patients [92.3%] continuing anti-IL5 therapy beyond 18 months. Renal function was preserved. One patient had MEPO switched to Rituximab to treat both EGPA and new onset rheumatoid arthritis. Three patients were switched to alternative anti-IL5 therapies, benralizumab (x2) and Reslizumab (x1).
Conclusion: The relapsing nature of EGPA places a potential dependency of therapy on steroids, underscoring the importance of pathway specific biologics to minimize exposure, prevent tissue damage and ensure early response to therapy. There was a 50% reduction in steroid dosage in this study, with longer-term anti-IL5 therapy continued in 12/13 patients due to clinical benefits achieved. Adjuvant immunotherapy is well tolerated and reduced in some cases.
References
- J.C.Jennette, et al 2012 Revised International Chapel Hil Consensus Conference Nomenclature of Vasculitides. 65, 1–11 (2013).
- Wechsler, M. E. et al. Mepolizumab or Placebo for Eosinophilic Granulomatosis with Polyangiitis. N. Engl. J. Med. 376, 1921–1932 (2017).
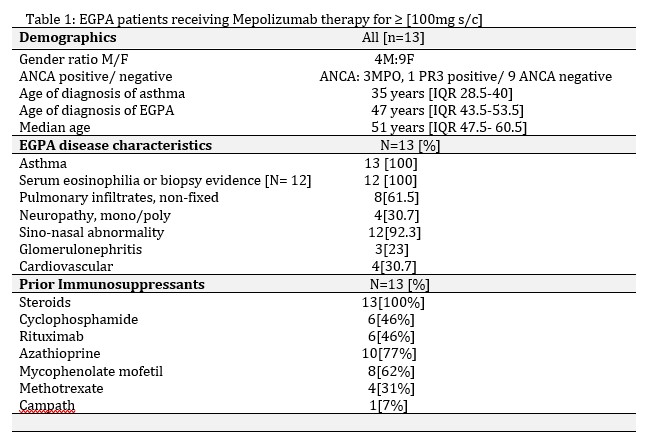 EGPA patients receiving anti-IL5 therapy for greater than 18 months
EGPA patients receiving anti-IL5 therapy for greater than 18 months
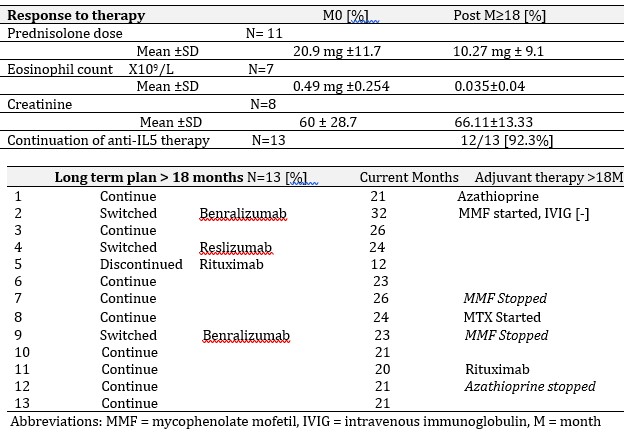 EGPA patients anti-IL5 therapy beyond eighteen months
EGPA patients anti-IL5 therapy beyond eighteen months
To cite this abstract in AMA style:
Egan A, Sivasothy P, Gore R, Owen C, Del Martinez Pero M, Jones R, Willcocks L, Smith R, Burns S, Jayne D. Anti-IL5 Therapy for Eosinophilic Granulomatosis with Polyangiitis (EGPA) – An 18 Month Follow-up Study as a Steroid Sparing Therapeutic Approach [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/anti-il5-therapy-for-eosinophilic-granulomatosis-with-polyangiitis-egpa-an-18-month-follow-up-study-as-a-steroid-sparing-therapeutic-approach/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/anti-il5-therapy-for-eosinophilic-granulomatosis-with-polyangiitis-egpa-an-18-month-follow-up-study-as-a-steroid-sparing-therapeutic-approach/

