Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: In SLE, persistent disease activity, disease flares and long-term glucocorticoid (GC) use all contribute to organ damage accrual. The effects of novel therapies on this trajectory are challenging to evaluate in clinical trials due to attrition of patients receiving SOC and limited study duration. The LASER study (NCT06485674) is an external control arm study assessing the effects of anifrolumab versus SOC on accumulation of long-term organ damage in patients with active SLE.
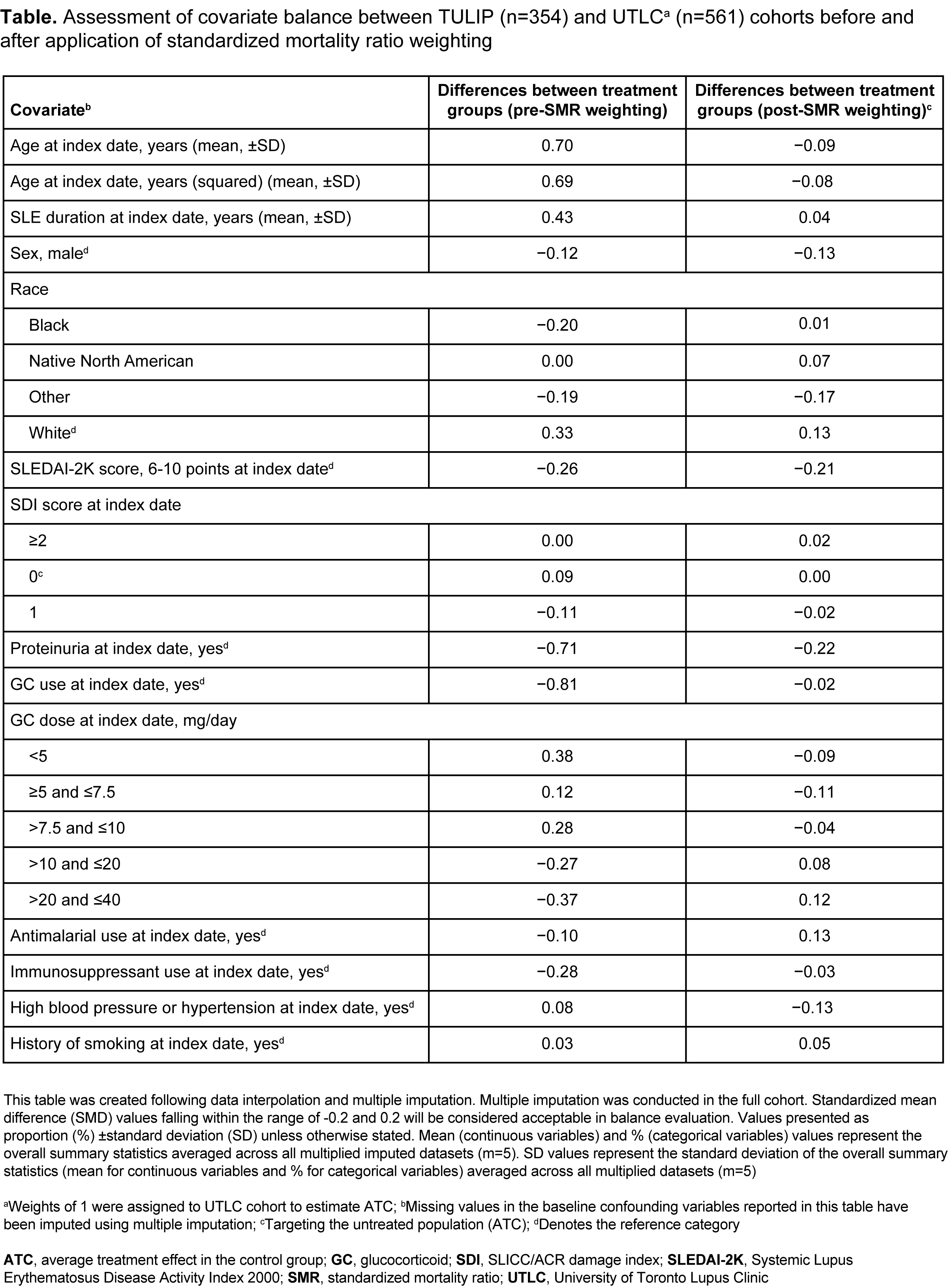
Methods: Patients assigned to anifrolumab 300 mg in the TULIP-1/-2 trials1 were included in the anifrolumab arm (n=354). The external control (SOC) arm included biologic-naïve patients from the University of Toronto Lupus Clinic (UTLC) cohort who matched key eligibility criteria from TULIP (n=561). Propensity score methods and censoring weighting were used to account for baseline confounding and loss to follow-up. Fourteen known predictors of organ damage were included in the propensity score model (Table). Treatment arms were considered balanced on a covariate if the standardized mean difference in propensity score was ≤0.2. The primary endpoint assessed the mean difference in SLICC/ACR Damage Index (SDI) changes from baseline at year 4 between the two arms, and the average treatment effect in the control group (ATC) was estimated using the SOC arm as the target group. Average treatment effect in the treated group (ATT) and 1:1 propensity score matching (PSM) were performed as sensitivity analyses. The secondary endpoint compared time to first organ damage (SDI) progression and was estimated in the ATC sample through Cox regression. SLEDAI-2K score and proteinuria remained unbalanced, and were included in the Cox regression model. ATC, ATT, and PSM data are mean (95% confidence interval [CI]) change in SDI.
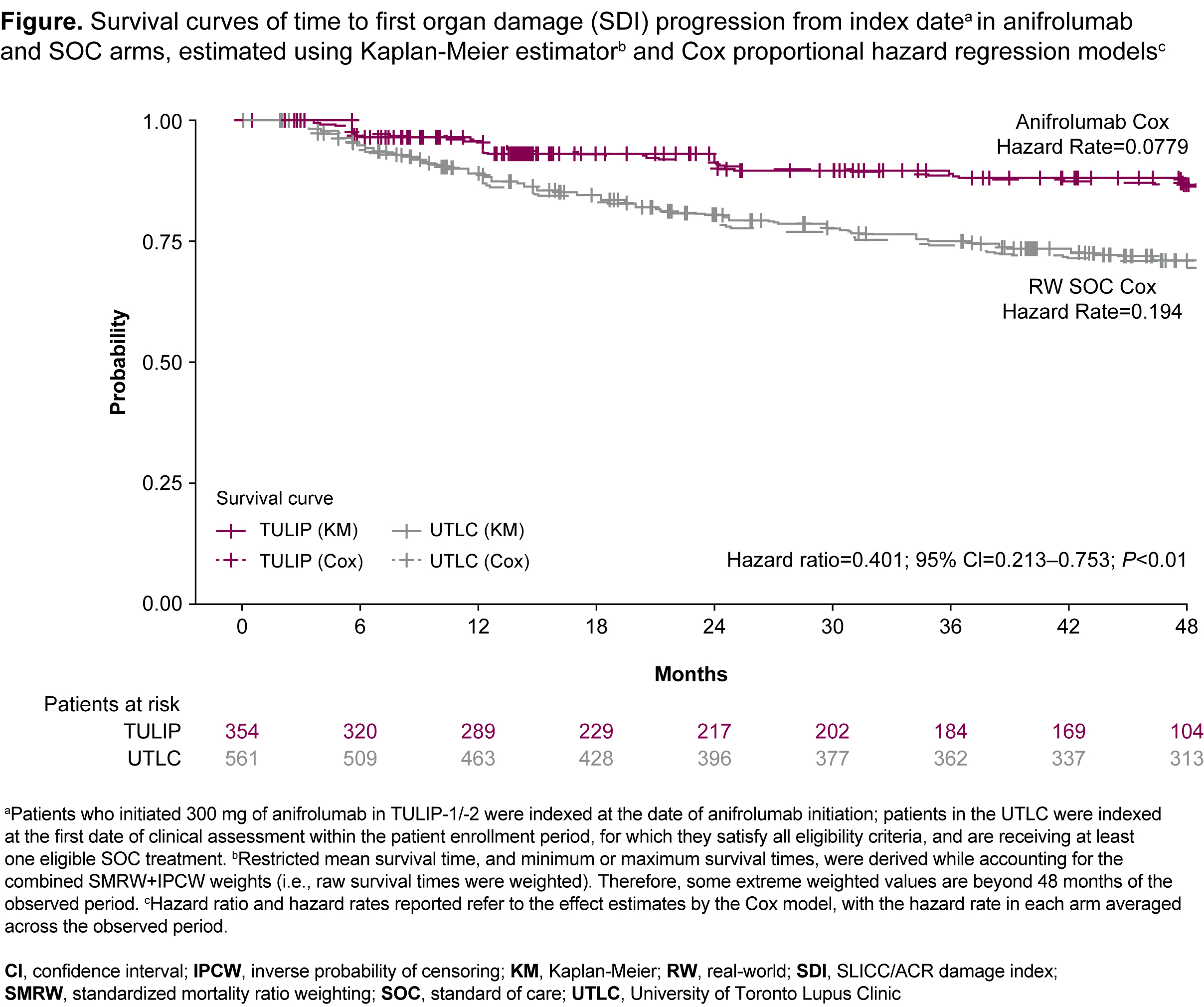
Results: All analyses indicated a greater increase in SDI with SOC compared with the anifrolumab arm after 4 years. In the ATC analysis (TULIP, n=354; UTLC, n=561), the mean (95% CI) change in SDI was 0.162 (0.046─0.279) with anifrolumab and 0.587 (0.479─0.695) with SOC; mean difference -0.416; P< 0.0001. In the ATT analysis (TULIP, n=354; UTLC, n=561), the mean (95% CI) change in SDI was 0.224 (0.149–0.299) with anifrolumab and 0.561 (0.390–0.733) with SOC; mean difference -0.337; P< 0.0001. The PSM (both TULIP and UTLC n=116) mean (95% CI) change in SDI was 0.201 (0.119–0.283) and 0.571 (0.380–0.761) in the anifrolumab and SOC arms; mean difference –0.370; P=0.002. Patients in the anifrolumab arm had 59.9% lower risk of first SDI progression over 48 months compared with SOC (hazard ratio=0.401; [95% CI: 0.213–0.753]; P<0.01) (Figure).
Conclusion: Our study suggests that long-term anifrolumab treatment may delay the onset of irreversible organ damage and reduce the rate of damage accrual in patients with moderate to severe SLE. The known benefits of anifrolumab in controlling disease activity, reducing flares, and reducing oral GC use in patients with SLE may help explain this observation.
Kalunian K, et al. Arthritis Rheumatol. 2023;75(2):253-265.
To cite this abstract in AMA style:
Touma Z, Bruce I, Furie R, Morand E, Tummala R, Chandran S, Abreu G, Knagenhjelm J, Arnold K, Lee H, Ralphs E, Kielar D, Bedenkov A, Waratani M. Anifrolumab Long-Term Treatment Is More Effective Against Organ Damage Than Standard of Care Alone: Results from an External Control Arm Study on Organ Damage in Phase 3 Clinical Trials and the University of Toronto Lupus Clinic Cohort [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/anifrolumab-long-term-treatment-is-more-effective-against-organ-damage-than-standard-of-care-alone-results-from-an-external-control-arm-study-on-organ-damage-in-phase-3-clinical-trials-and-the-univer/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/anifrolumab-long-term-treatment-is-more-effective-against-organ-damage-than-standard-of-care-alone-results-from-an-external-control-arm-study-on-organ-damage-in-phase-3-clinical-trials-and-the-univer/