Session Information
Date: Monday, October 27, 2025
Title: (1517–1552) Systemic Lupus Erythematosus – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Anifrolumab (ANI) is a human monoclonal antibody that binds to the type I interferon receptor subunit 1 (IFNAR1). ANI was approved by Spanish authorities for the treatment of adults with moderate to severe systemic lupus erythematosus (SLE) with positive autoantibodies, in combination with standard treatment.In patients with SLE treated with ANI in clinical practice in Spain, our aim was to assess a) the profile of patients receiving ANI b) effectiveness and c) safety.
Methods: Observational multicenter study of patients diagnosed with SLE (EULAR/ACR 2019 classification criteria) treated with ANI. Data were collected from medical records up to April 31st 2025. Demographic, clinical, laboratory, and pathologic variables were evaluated, along with previous and concomitant therapies, disease activity indices (SLE-DAS, SLEDAI-2K, PGA), organ damage index (SDI), and safety were evaluated.
Results: 206 patients (183 females/23 males), mean age 44.6 ± 12.6 years (range 15-90 years) (54 hospitals) were included. Baseline characteristics of patients and prior treatment before starting ANI are summarized in TABLE.The main reason for starting ANI was clinical activity: cutaneous (n=124, 61.7%), articular (n=98, 48.5%), hematological (n=55, 27.2%), corticosteroid dependence (n=11, 5.4%), renal (n=8, 4%), serositis (n=6, 3%), serious adverse events with other immunosuppressants (n=4, 1.9%), neurological (n=2, 1%), and Kikuchi disease (n=1, 0.5%).The mean number of immunosuppressants (synthetic/biologic) received prior to ANI was 2.5±1.6 (range 0-8). All patients received 300 mg/4 weeks of ANI except two patients with renal involvement who received a loading dose (900 mg/4 weeks x3 months followed by 300 mg/4 weeks). In addition to corticosteroids and antimalarials, ANI was administered in many cases concomitantly with other immunomodulator drug (TABLE).A rapid (from the 1st month) and maintained significant improvement was observed in: a) disease activity (SLE-DAS, SLEDAI-2K, PGA) (Figure 1) b) immunologic markers (decrease in anti-dsDNA antibody titers and normalization of C3 and C4 levels) (Figure 1) and c) prednisone dose (Figure 2). The organ damage index remained stable.After a mean follow-up of 7.5 ± 5.3 months, a reduction in the number of relapses was observed, from a median [IQR] of 2 [1-6] prior ANI to 0 [0-0] after ANI (p < 0.001). The most relevant adverse events were: herpes zoster (n=5), headache (n=3), upper respiratory tract infection (n=3), pneumonia (n=3), arterial hypotension (n=2), hidradenitis suppurativa (n=2) and vaginal candidiasis (n=2). In the follow-up, 20 patients discontinued treatment due to primary failure (n=8), secondary failure (n=3), severe infections (n=6), hypotension (n=1), acute anterior uveitis (n=1), and pregnancy desire (n=1).
Conclusion: As in clinical trials, a rapid and maintained effectiveness was observed; even in severe and refractory patients. ANI was used in monotherapy or combined with other immunosuppressive drugs, including other biological therapies. The safety profile was acceptable with the limitation of short follow-up.
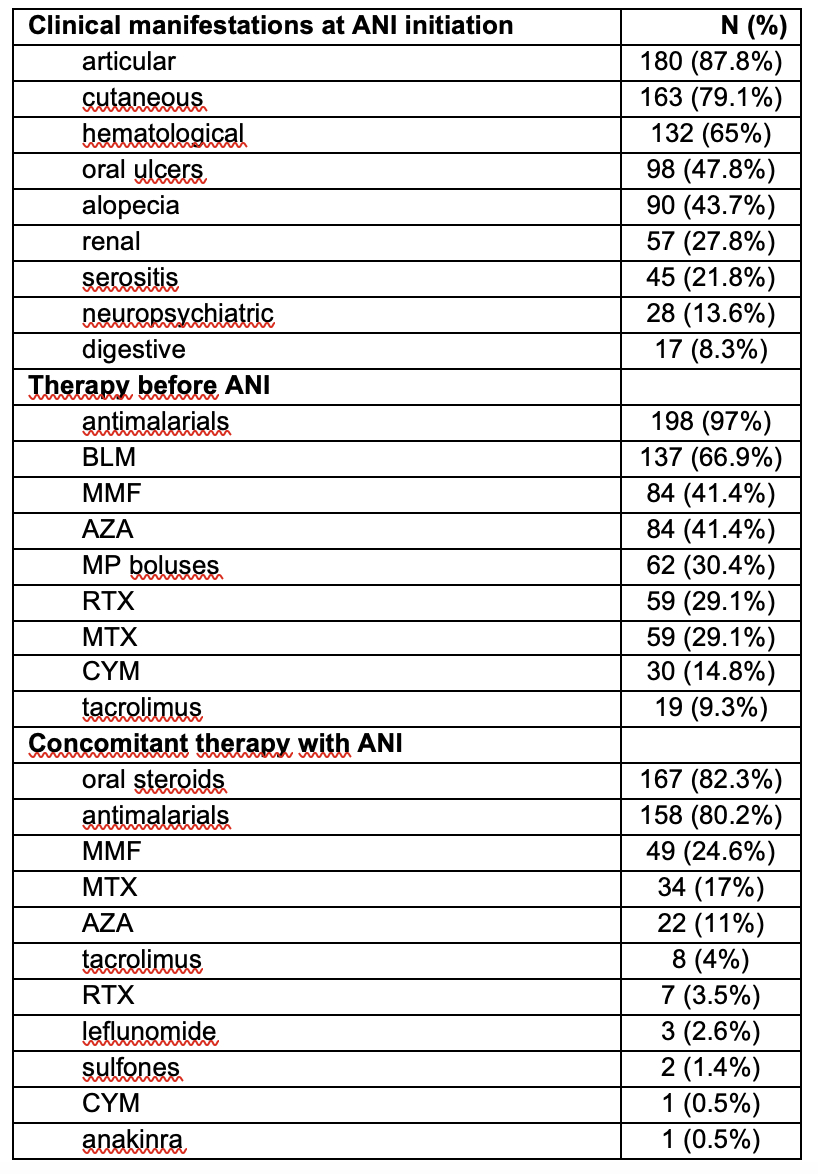 TABLE. Clinical manifestations and treatments received before starting anifrolumab
TABLE. Clinical manifestations and treatments received before starting anifrolumab
Abbreviations in alphabetical order: ANI: anifrolumab; AZA: azathioprine; BLM: belimumab; CsA: cyclosporine; CYM: cyclophosphamide; MMF: mycophenolate mofetil; MP: methylprednisolone; MTX: Methotrexate; RTX: rituximab
.jpg) Figure 1. Evolution of C3, C4 and anti-dsDNA levels and activity and organ damage indices after starting anifrolumab.
Figure 1. Evolution of C3, C4 and anti-dsDNA levels and activity and organ damage indices after starting anifrolumab.
.jpg) Figure 2. Reduction in prednisone dosage after initiation of anifrolumab.
Figure 2. Reduction in prednisone dosage after initiation of anifrolumab.
Disclosures: V. Calvo-Río: AstraZeneca, 6, MSD, Roche, AbbVie, Lilly, Celgene, Grünenthal, and UCB Pharma, 2, 6; C. Secada-Gómez: None; M. Retuerto Guerrero: None; J. Font-Urgelles: None; I. Casafont-Solé: None; A. Mayo-Juanatey: None; J. Alegre-Sancho: None; D. FREITES: None; C. Hormigos Martin: None; N. Garrido: None; G. GONZALEZ ARRIBAS: None; J. Miguelez Sanchez: None; A. García-Valle: None; M. Ibañez: None; F. Lozano Morillo: None; Á. García Manzanares: None; S. Sandoval-Moreno: None; J. Cortés-Hernández: AstraZeneca, 6, Bristol-Myers Squibb (BMS), 1, GlaxoSmithKline (GSK), 6, Novartis, 1, 5, 6; D. Palma-Sanchez: None; L. LOJO-OLIVEIRA: None; E. Cervantes: None; P. Collado Ramos: None; c. arciniega Larios: None; L. Sala: None; E. Labrador-Sánchez: None; C. Peralta-Ginés: None; N. Plaza-Aulestia: None; I. Urionaguena Onaindia: None; M. Medina Malone: None; J. Rosas Gómez de Salazar: None; M. Corteguera: None; L. Cebrian: None; F. Anton Pages: None; J. Lamua-Riazuelo: None; M. Fábregas Canales: None; M. Alados Hernández: None; M. Garijo Bufort: None; A. Pamies: None; L. Sarabia: None; R. Aguirre-del-Pino: None; J. Cabezas Lefler: None; a. seijas: None; C. Carrasco-Cubero: None; A. López-Cerón Cofiño: None; V. Ortiz-Santamaria: None; S. Castañeda: None; M. Laino: None; C. Ordás Calvo: None; C. arconada: None; a. Urruticoechea-Arana: None; B. Garcia-Magallon: AstraZeneca, 6, 12; A. Acosta Alfaro: None; S. Leal Rodriguez: None; M. Salido Olivares: None; P. Lavilla Villar: None; A. Brandy: None; I. Ros Vilamajo: None; A. García Martos: None; B. Magallares: None; G. Sada Urmeneta: None; C. Córdoba Martín: None; E. Riera Alonso: None; C. Bejerano-Herreria: None; R. Blanco: AbbVie/Abbott, 2, 5, 6, Bristol-Myers Squibb (BMS), 5, 6, Eli Lilly, 5, 6, Janssen, 5, 6, Merck/MSD, 2, 5, 6, Pfizer, 2, 6, Roche, 2, 5, 6.
To cite this abstract in AMA style:
Calvo-Río V, Secada-Gómez C, Retuerto Guerrero M, Font-Urgelles J, Casafont-Solé I, Mayo-Juanatey A, Alegre-Sancho J, FREITES D, Hormigos Martin C, Garrido N, GONZALEZ ARRIBAS G, Miguelez Sanchez J, García-Valle A, Ibañez M, Lozano Morillo F, García Manzanares Á, Sandoval-Moreno S, Cortés-Hernández J, Palma-Sanchez D, LOJO-OLIVEIRA L, Cervantes E, Collado Ramos P, arciniega Larios c, Sala L, Labrador-Sánchez E, Peralta-Ginés C, Plaza-Aulestia N, Urionaguena Onaindia I, Medina Malone M, Rosas Gómez de Salazar J, Corteguera M, Cebrian L, Anton Pages F, Lamua-Riazuelo J, Fábregas Canales M, Alados Hernández M, Garijo Bufort M, Pamies A, Sarabia L, Aguirre-del-Pino R, Cabezas Lefler J, seijas a, Carrasco-Cubero C, López-Cerón Cofiño A, Ortiz-Santamaria V, Castañeda S, Laino M, Ordás Calvo C, arconada C, Urruticoechea-Arana A, Garcia-Magallon B, Acosta Alfaro A, Leal Rodriguez S, Salido Olivares M, Lavilla Villar P, Brandy A, Ros Vilamajo I, García Martos A, Magallares B, Sada Urmeneta G, Córdoba Martín C, Riera Alonso E, Bejerano-Herreria C, Blanco R. . Anifrolumab In Systemic Lupus Erythematosus. Spanish Multicenter Registry In Clinical Practice [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/anifrolumab-in-systemic-lupus-erythematosus-spanish-multicenter-registry-in-clinical-practice/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/anifrolumab-in-systemic-lupus-erythematosus-spanish-multicenter-registry-in-clinical-practice/
