Session Information
Date: Monday, November 18, 2024
Title: SLE – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
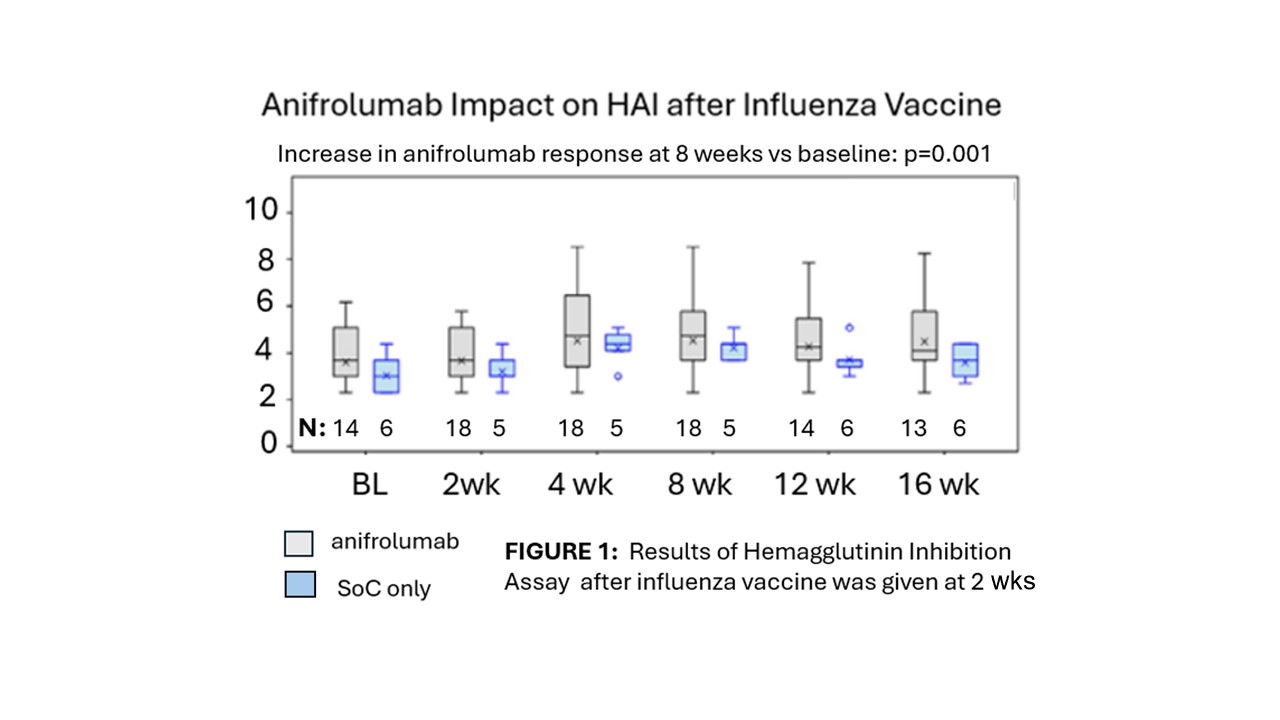
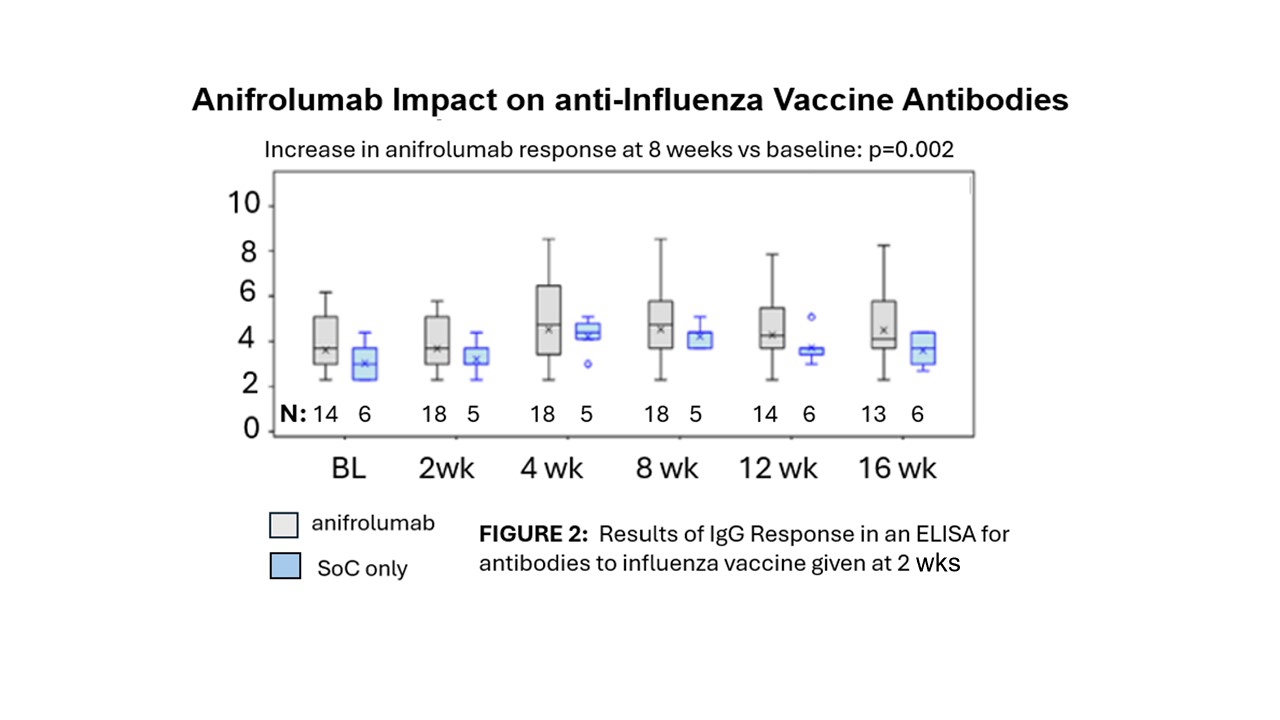
Background/Purpose: Risk for infections in systemic lupus may arise from immunosuppressant treatments or intrinsic immune defects. Disordered interferon signals are a hallmark of SLE. Anifrolumab, which targets the Type I Interferon Receptor (IFNAR), has been found to be safe and effective but, not surprisingly, inhibition of interferon signals is associated with some viral infections and herpes zoster reactivation. We previously reported a relationship between interferon activation and suppressed response to influenza vaccine. The current study examined hemagglutinin inhibition and anti-influenza vaccine antibodies after the administration of flu vaccine in SLE patients on anifrolumab.
Methods: Between 2020 and 2023, 18 patients with active, moderate to severe SLE received 3 monthly doses of open label anifrolumab 300 mg IV. Two weeks after dose 1, an FDA-approved quadrivalent, season-specific influenza vaccination was given, In the third season (2022-23), 6 additional SLE patients participated in the protocol without receiving anifrolumab. All patients continued standard of care treatment (SoC). Vaccine response was quantified with a hemagglutinin inhibition assay (HAI) using predominate antigen of active strains each year, and results of an enzyme-linked immunosorbent assay (ELISA) measuring IgG to the relevant seasonal vaccine antigens. Comparison of responses before and after vaccination and in patients who did or did not receive anifrolumab was performed by non-parametric testing. The proportion in each group developing a twofold increase in values for each test at week 8 compared to baseline, utilized Fisher’s Exact Test. Confidence intervals were derived with the Exact Clopper-Pearson Method.
Results: In a combined analysis merging data from all three years, anifrolumab-treated patients had no observable differences in vaccine response by either HAI (fig 1) or by antibody levels to the vaccine (fig 2). At week 8, geometric mean titers (GMTs) and geometric standard deviations (GSDs) for HAI were anifrolumab: 123.0 (5.39) and control: 69.6 (1.79). GMTs (GSDs) for anti-vaccine antibody concentrations were anifrolumab: 171.8 (3.59) μg/mL and control: 151.7 (2.62). Geometric mean fold rises (GMFRs) (GSDs) of HAI titers from baseline to week 8 were anifrolumab 1.6 (4.52), control: 3.0 (1.46) and GMFRs of anti-influenza IgG were anifrolumab: 1.5 (2.91) and control: 2.8 (3.61) No differences were noted when vaccine responses were evaluated separately for each influenza season. All 6 patients in the control group and 15 (78.9%) of patients in the anifrolumab group developed at least one adverse event (AE) during the study. All AEs were mild or moderate in intensity. There were no serious adverse events, deaths, adverse events of special interest, or adverse events leading to discontinuation of treatment.
Conclusion: Humoral antibody responses induced by seasonal influenza virus vaccination in adult SLE patients with SLE were comparable between patients receiving anifrolumab and those only receiving standard of care, Anifrolumab was well tolerated with no unexpected safety findings in the context of influenza vaccination.
To cite this abstract in AMA style:
Arriens C, Askanase A, Machua W, Koumpouras F, Smith K, Guthridge J, James J, Merrill J. Anifrolumab Effects on Response to Influenza Vaccine in SLE [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/anifrolumab-effects-on-response-to-influenza-vaccine-in-sle/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/anifrolumab-effects-on-response-to-influenza-vaccine-in-sle/