Session Information
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: The management of ANCA-associated vasculitis (AAV) evolved substantially in recent years because of evidence supporting the efficacy of various treatment regimens. As such, treatment approaches may vary across physicians. Understanding current practice can guide the design of pragmatic trials, inform cost-effectiveness research, and establish benchmarks. We sought to identify AAV treatment patterns in the US, especially outside of referral centers.
Methods: AAV patients seen in practices participating in the Rheumatology Informatics System for Efficacy (RISE) registry between January 1st, 2015 and December 31st, 2017 were included. AAV was defined as ≥ physician visit-associated 2 ICD-10 codes for AAV (M31.1, M31.31, M31.7, M30.1) ≥ 3 months apart and prescription/administration for rituximab (RTX), cyclophosphamide (CYC), azathioprine (AZA), methotrexate (MTX), mycophenolate mofetil (MMF), or glucocorticoids 3 months before or up to 6 months after the first ICD-10 code. New or flaring AAV cases were identified based on encounter codes for New/Consult visit; medications prescribed within 3 months of the 1st ICD-10 diagnosis code were assumed to be for “induction therapy.” Demographic and prescription trends were assessed overall and across US regions. This data was supported by the ACR’s RISE Registry. However, the views expressed represent those of the authors, not necessarily those of the ACR.
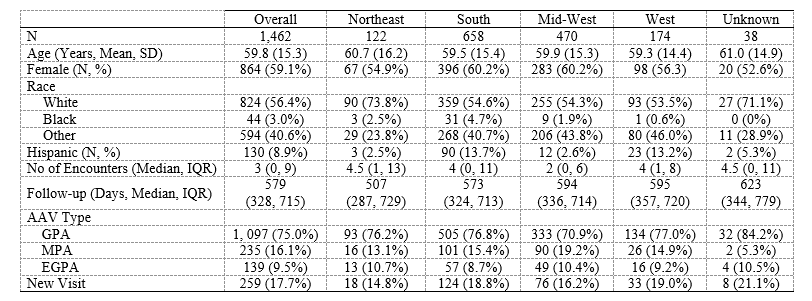
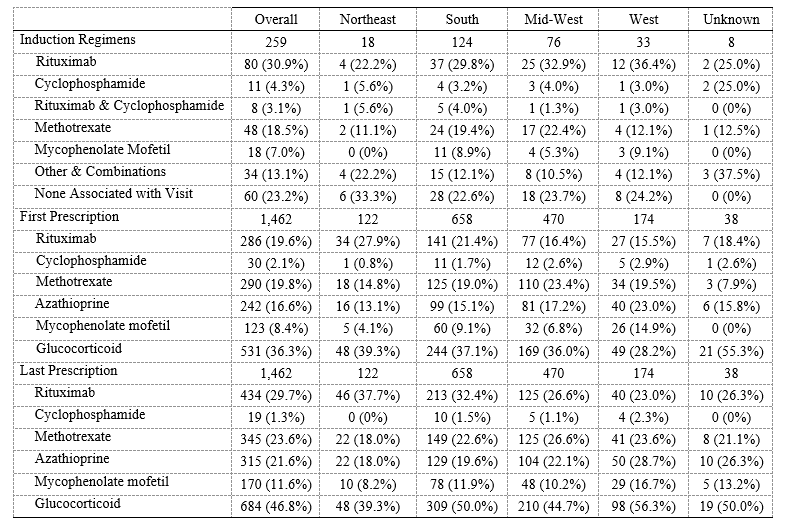
Results: 1,462 patients fulfilled the AAV case definition; 259 (18%) were new cases (Table 1). GPA was more common (1,097, 75%) than MPA (235, 16%) or EGPA (139, 10%). The mean age at the initial visit was 59.8 (±15.3) and 864 (59%) were female. Most visits occurred in the South (658, 45%) followed by the Mid-West (470, 32%), West (174, 12%), and Northeast (122, 8%). During the study period, patients had a median of 3 (IQR 0, 9) rheumatology clinician visits and an average follow-up of 579 days (IQR 328, 715). Among new patients, the most common induction regimens (Table 2) were RTX (80, 31%) followed by MTX (48, 19%). CYC was used in 11 (4%) cases. Among all patients (new and follow-up), the most common initially prescribed medication was a glucocorticoid (531, 36%), followed by MTX (290, 20%), RTX (286, 20%), and/or AZA (242, 17%); the most commonly prescribed medication at the end of follow-up was a glucocorticoid (684, 46.8%), followed by RTX (434, 30%), MTX (345, 24%), AZA (315, 22%), and/or MMF (170, 12%). Prescription trends were similar across regions.
Conclusion: This is among the first US studies to evaluate demographic and management patterns of AAV outside of major referral centers. The demographics of AAV patients in RISE are similar to those of other AAV cohorts. There was wide variation in management at different time points in care but these variations were similar across regions. Though RTX and CYC are considered non-inferior to one another, there was minimal CYC use. Glucocorticoids, RTX, and MTX were the most commonly prescribed medication throughout AAV care. Despite the superiority of RTX over AZA for maintenance of remission in AAV, AZA use was common. Future studies should characterize the severity of AAV cases in RISE and evaluate patient and provider treatment preferences and practice variability.
To cite this abstract in AMA style:
Wallace Z, Yun H, Curtis J, Yang S, Chen L, Stone J, Choi H. ANCA-Associated Vasculitis Management in the United States: Data from the RISE Registry [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/anca-associated-vasculitis-management-in-the-united-states-data-from-the-rise-registry/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/anca-associated-vasculitis-management-in-the-united-states-data-from-the-rise-registry/