Session Information
Date: Sunday, November 5, 2017
Title: Rheumatoid Arthritis – Clinical Aspects Poster I: Treatment Patterns and Response
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Biosimilar infliximab (CT-P13) was first approved in Europe in 2013.This study compared treatment (tx) patterns of Turkish pts with a diagnosis of rheumatoid arthritis(RA) who initiated originator IFX(IFX) & either continued IFX or switched to CT-P13.
Methods: Adult pts with ≥1 RA diagnosis code & IFX claim were identified in a national Turkish healthcare database. Eligible pts initiated & continued IFX (Continuer cohort; CC) or initiated IFX & switched to CT-P13 (Switch cohort; SC) during the study period (01DEC2010-01JUN2016). The index date was defined as CT-P13 switch date for SC or a random IFX date during period of CT-P13 availability for CC. Cohorts were matched on age,sex,&number of IFX prescriptions during baseline(BL). Discontinuation (d/c) was defined as a switch to another biologic or no index biologic for ≥120 days without censoring. Pt demographics, d/c & switching were summarized with descriptive statistics.
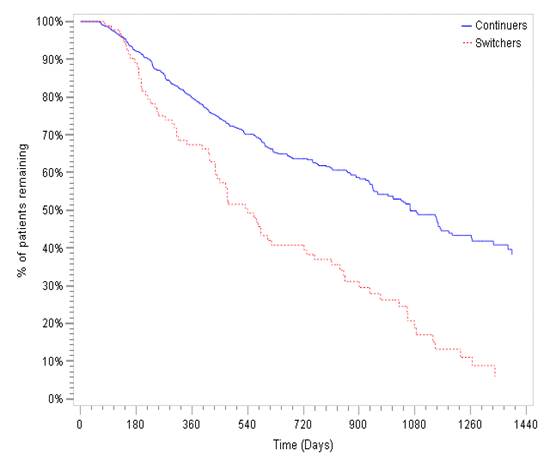
Results: A total of 697 pts initiating IFX were studied; 87%(N=605) continued IFX throughout the study period; 13%(N=92) switched to CT-P13. BL & clinical characteristics are shown in Table. Mean duration of IFX therapy during BL period was 422 days(CC) & 438 days(SC). Average duration of post-index follow-up was 16 months(CC) & 15 months(SC). During the combined BL & post-index periods, median time on any IFX therapy was 1080 days(CC)&540 days(SC)(Figure).D/c post-index occurred in 19%(CC) & 87%(SC); mean time from index to IFX d/c /censoring was 276 days(CC) while mean time from index to CT-P13 d/c /censoring was 132 days.While switching from IFX to CT-P13 occurred in 13% all IFX initiators on the index date; an additional 10% of the CC switched to a non-IFX anti-TNF post-index. The majority of SC(82%) switched again post-index (off CT-P13) & 88% of those re-initiated IFX. Regional variation in switching was noted. Switching from IFX to CT-P13 occurred most frequently in Central Anatolia (26% of 154 IFX initiators).Switching from CT-P13 occurred in >75% of SC pts in all regions except for Aegean(44% switched from CT-P13 to another biologic, predominantly IFX).
Conclusion: In Turkey, RA pts maintained on IFX had greater tx persistence than those who initiated IFX & switched to CT-P13. CT-P13 d/c resulted in IFX re-initiation in the majority of pts. Reasons for d/c are unknown, however regional differences in practice patterns were observed.
|
Table Demographics and Treatment Patterns for Continuer and Switcher Cohorts |
||||
|
|
Continuers Cohort |
Switchers Cohort |
||
|
(N=605) |
(N=92) |
|||
|
N/Mean |
%/SD |
N/Mean |
%/SD |
|
|
Age (Mean) (years) |
41 |
10.3 |
43 |
11.8 |
|
Sex |
||||
|
Women |
309 |
51% |
48 |
52% |
|
Baseline Concomitant Disease |
||||
|
Ankylosing Spondylitis |
102 |
18% |
27 |
13% |
|
Psoriatic Arthritis |
48 |
8% |
11 |
5% |
|
Crohn’s Disease |
244 |
42% |
114 |
56% |
|
Ulcerative Colitis |
42 |
7% |
9 |
4% |
|
Average Length of Follow-up Period (months) |
16 |
2 |
15 |
2 |
|
Post-Index Concomitant Disease |
||||
|
Ankylosing Spondylitis |
363 |
60% |
58 |
63% |
|
Psoriatic Arthritis |
102 |
17% |
13 |
14% |
|
Crohn’s Disease |
48 |
8% |
3 |
3% |
|
Ulcerative Colitis |
33 |
5% |
4 |
4% |
|
Switching |
||||
|
N and % of patients with ≥1 switch |
115 |
19% |
75 |
82% |
|
N and % Primary Switches from CT-P13 to IFX |
NA |
NA |
66 |
88% |
|
Geographical Distribution of Patients with ≥ 1 switches (n=115 vs. 75) |
||||
|
East Anatolia |
6 |
5% |
1 |
1% |
|
South Eastern Anatolia |
10 |
9% |
9 |
12% |
|
Marmara |
39 |
34% |
9 |
12% |
|
Aegean |
4 |
3% |
4 |
5% |
|
Mediterrane |
19 |
17% |
16 |
21% |
|
Black sea |
9 |
8% |
1 |
1% |
|
Central Anatolia |
28 |
24% |
35 |
47% |
|
Discontinuation |
||||
|
N of Patients with Confirmed Discontinued |
205 |
34% |
80 |
87% |
|
Time to confirmed discontinuation (days) |
117 |
78 |
98 |
60 |
|
Time to any discontinuation or censoring (days) |
276 |
124 |
132 |
104 |
N: Number; %: Percentage; IFX: Infliximab; SD: Standard Deviation; NA: Not available.
Figure Kaplan Meier (KM) curve for any Infliximab Use for the Switcher and Continuers Cohorts during the Baseline and Follow up Period.
To cite this abstract in AMA style:
Ellis LA, Simsek I, Xie L, Ogbomo A, Parenti D, Goyal K, Yazici Y. Analysis of Real-World Treatment Patterns in a Matched Sample of Rheumatology Patients with Continuous Infliximab Therapy or Switched to Biosimilar Infliximab [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/analysis-of-real-world-treatment-patterns-in-a-matched-sample-of-rheumatology-patients-with-continuous-infliximab-therapy-or-switched-to-biosimilar-infliximab/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/analysis-of-real-world-treatment-patterns-in-a-matched-sample-of-rheumatology-patients-with-continuous-infliximab-therapy-or-switched-to-biosimilar-infliximab/