Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose
Certolizumab pegol (CZP) is approved for adult patients (pts) with rheumatic diseases (RA, PsA and AS) at a maintenance dose of 200mg every 2 wks (Q2W) or 400mg every 4 wks (Q4W). In RAPID11 (52 wks) and RAPID22 (24 wks) randomized clinical trials (RCTs; NCT00152386 and NCT00160602), CZP was administered at a loading dose of 400mg at Wks 0, 2, and 4, followed by CZP 200mg Q2W, or at CZP 400mg Q2W in pts with active RA. All pts entering open-label extensions (OLEs; NCT00175877 and NCT00160641) received CZP 400mg Q2W for ≥6 months. The dose was subsequently reduced to CZP 200mg Q2W for all pts3,4 as feeder studies revealed that the null hypothesis of no difference between doses could generally not be rejected. We present safety data evaluating the potential effect of CZP dose change over 12 wks prior to and post dose-escalation or dose-reduction, in line with treat-to-target.
Methods
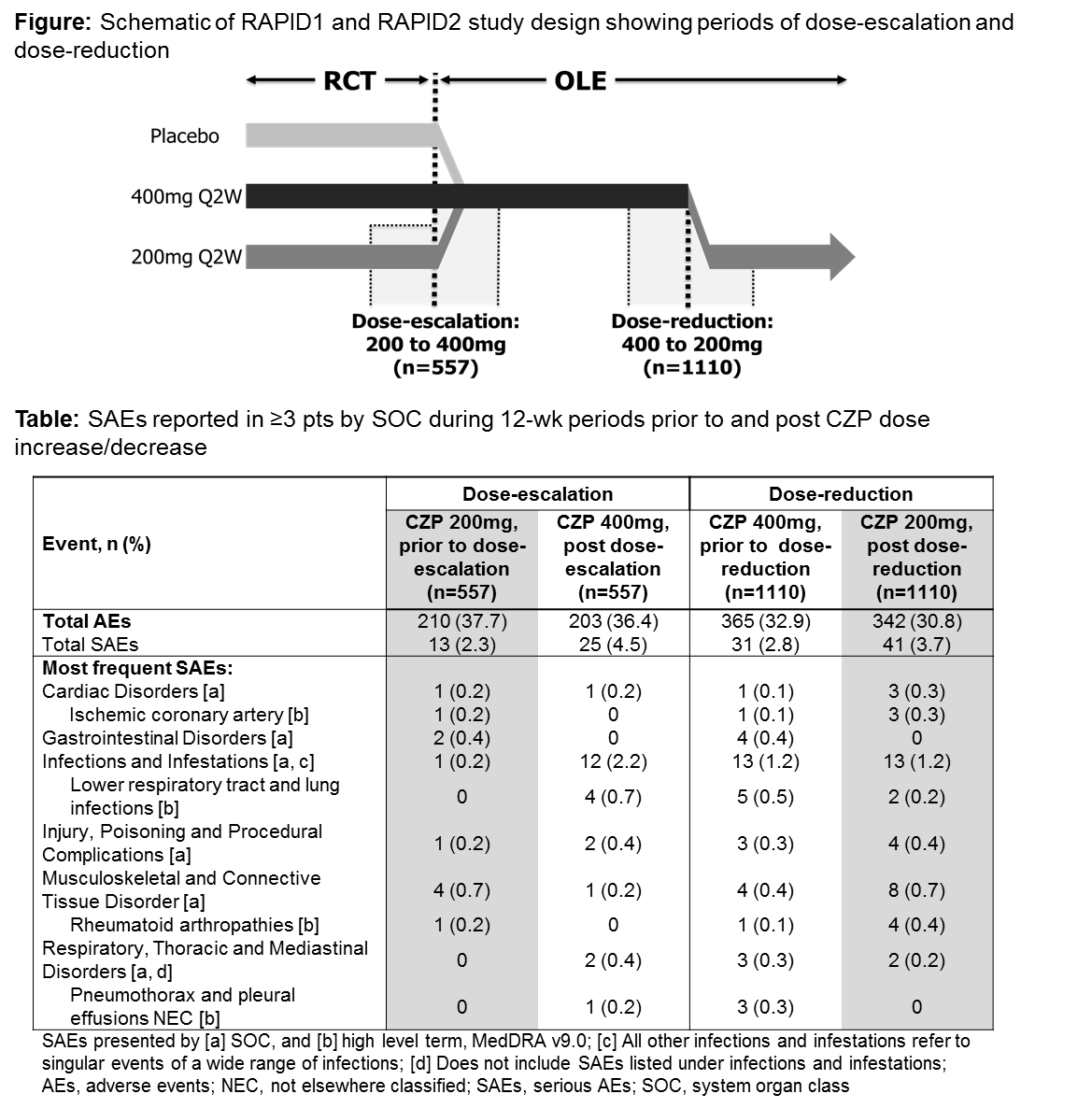
Post-hoc analysis was performed on pooled RAPID1 and RAPID2 CZP data: 1) dose-escalation from CZP 200mg Q2W to CZP 400mg Q2W, 2) dose-reduction from CZP 400mg Q2W to CZP 200mg Q2W (Figure).
Results
557 pts randomized to CZP 200mg Q2W in the RCTs entered the OLEs, with dose-escalation to 400mg Q2W. Of these, 210 pts (37.7%) experienced an adverse event (AE) in the 12 wks prior to dose-escalation versus 203 pts (36.4%) in the 12 wks post dose-escalation (Table). 94.4% of AEs were mild-moderate: 65 and 71 pts, respectively, experienced AEs considered to be drug-related. Serious AEs are reported in the table. The incidence of infections increased post dose-escalation. Malignancies were reported in 3 pts during 12 wks prior to (1 testis, 2 basal cell carcinoma [BCC]) and post (testis, lung, and peritoneal) dose-escalation. During OLEs, 1110 pts received CZP 400mg Q2W for ≥6 months before dose-reduction. Of these, 365 pts (32.9%) experienced an AE in the 12 wks prior to dose-reduction versus 342 pts (30.8%) in the 12 wks post dose-reduction; 94.1% of AEs were mild-moderate: 102 and 91 pts, respectively, experienced AEs considered drug-related. Incidence of SAEs and infections was similar prior to and post dose-reduction. 2 pts reported malignancies in the 12 wks post dose-reduction (1 gastric, 1 BCC). 2 cases of opportunistic infection (including tuberculosis) were reported in the 12 wks post dose-reduction. Overall, the number of AEs leading to withdrawals was low and no deaths were reported during the dose-escalation and dose-reduction study periods evaluated.
Conclusion
Overall, despite a modest infection rate increase after dose-escalation, no new safety concerns emerged during dose-escalation or dose-reduction, and AE rates were generally similar between periods. However, the natural trend of a decreasing rate of AEs was observed over time, as is usual in RA clinical trials.
References
1. Keystone E. Arthritis Rheum 2008;58:3319-3329
2. Smolen J.S. Ann Rheum Dis 2009;68:797-804
3. Keystone E. Ann Rheum Dis 2013;epub
4. Smolen J.S. Arthritis Rheum 2013;65:S988
Disclosure:
B. Haraoui,
Abbott, Amgen, BMS, Janssen, Pfizer, Roche and UCB Pharma,
2,
Abbott, Amgen, BMS, Janssen, Pfizer, Roche and UCB Pharma,
8,
Abbott, Amgen, BMS, Janssen, Pfizer, Roche and UCB Pharma,
5;
V. P. Bykerk,
None;
R. van Vollenhoven,
AbbVie, BMS, GSK, Pfizer, Roche, UCB,
2,
AbbVie, Biotest, BMS, GSK, Janssen, Lilly, Merck, Pfizer, Roche, UCB, Vertex,
5;
M. de Longueville,
UCB Pharma,
3;
K. Luijtens,
UCB Pharma,
3;
P. Ralston,
UCB Pharma,
5;
A. Kavanaugh,
Abbott, Amgen, BMS, Pfizer, Roche, Janssen, UCB Pharma,
2.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/analysis-of-pooled-data-from-two-randomized-controlled-trials-and-their-open-label-extensions-long-term-safety-in-rheumatoid-arthritis-before-and-after-certolizumab-pegol-dose-increasedecrease/