Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose
Active RA significantly impairs health-related quality of life (HRQoL) and physical function of patients. Granulocyte-macrophage colony-stimulating factor (GM-CSF) plays a key role in macrophage activation and RA pathogenesis, including inflammatory and arthritic pain development. A prior Phase 2a study (EARTH; NCT01050998) in active RA showed that mavrilimumab produced clinically meaningful improvements across a variety of patient-reported outcomes (PROs). Here we assess the benefit experienced by patients in a 24-week Phase 2b study.
Methods
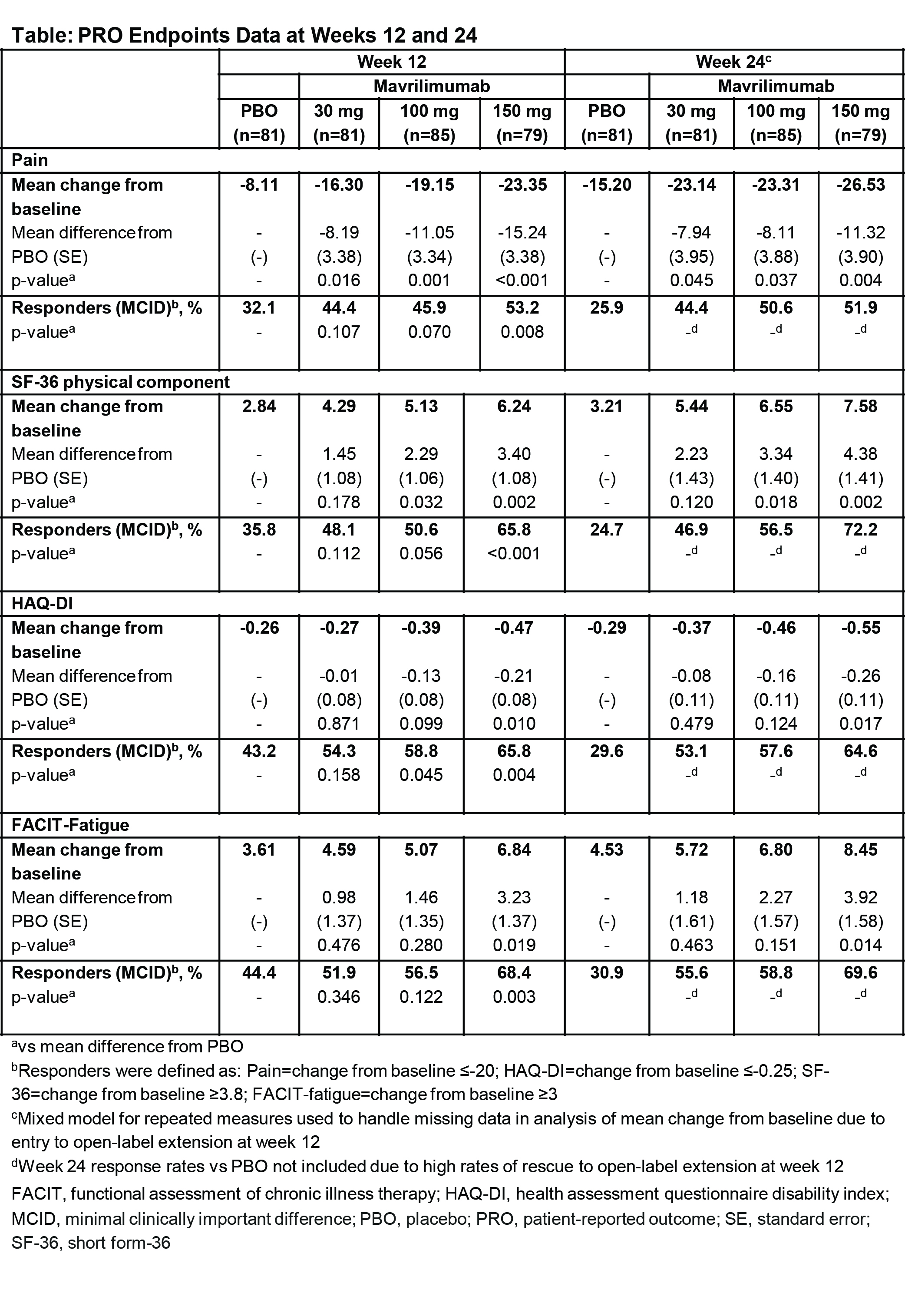
This randomized, placebo (PBO)-controlled, multicenter study (NCT01706926) evaluated the efficacy/safety of 3 subcutaneous mavrilimumab doses vs PBO every 2 weeks (Q2W) over 24 weeks. Patients with adult-onset RA (18–80 years; DAS28-CRP ≥3.2; ≥4 swollen joints; inadequate response to ≥1 DMARD; receiving methotrexate) were enrolled. Co-primary endpoints were changes in DAS28-CRP score (day 1 to week 12) and ACR20 response (week 24). PRO endpoints included changes from baseline and percent responders in patient assessments of pain, HRQoL (SF-36), physical function (HAQ-DI), and fatigue (FACIT–Fatigue). Patients could enter a long-term open-label rescue study from week 12 (not reported).
Results
In total, 326 patients (mean [SD] age 51.8 [11.1] years; female 86.5%) with a mean (SD) DAS-CRP of 5.8 (0.9) and DAS28-ESR of 6.6 (0.9) were randomized to 30, 100, or 150 mg mavrilimumab, or PBO (n=81, 85, 79 and 81, respectively). Both co-primary endpoints (DAS28-CRP at week 12; ACR20 at week 24) were met at all mavrilimumab doses. At weeks 12 and 24, mavrilimumab treatment groups showed improvements in PROs vs PBO (Table), with more than half of patients receiving mavrilimumab 150 mg having a clinically meaningful response. At this dose, significant improvements (p=0.017–p<0.001) were seen vs PBO across multiple PRO endpoints at weeks 12 and 24 (Table). Statistically significant improvements in pain were seen for mavrilimumab from week 1. Of mavrilimumab 30, 100, and 150 mg patients with a pain response at week 12, 72.2% (26/36), 69.2% (27/39), and 66.7% (28/42) maintained responses at week 24. HAQ-DI responses at week 12 were maintained at week 24 in 75.0% (33/44), 84.0% (42/50), and 78.8% (41/52) patients (mavrilimumab 30, 100 and 150 mg, respectively).
Conclusion
Targeting activated macrophages through inhibition of the GM-CSFRα pathway with mavrilimumab, especially at a dose of 150 mg Q2W, substantially and rapidly reduced RA disease activity. In turn, patients receiving mavrilimumab, vs PBO, reported significant and clinically meaningful sustained improvements in multiple PROs that reflect patients’ pain, HRQoL, physical function and fatigue. The majority of mavrilimumab patients with improvement in pain and physical function at week 12 sustained these improvements to the end of the study at week 24.
Disclosure:
J. M. Kremer,
Corrona,
1,
AbbVie, Amgen, Genentech, Lilly, Pfizer,
2,
AbbVie, Amgen, Genentech, Lilly, Pfizer, BMS,
5,
Corrona,
3;
G. Burmester,
Medimmune,
5;
M. Weinblatt,
BMS, UCB, Crescendo Bioscience,
2,
Medimmune, AstraZeneca, Amgen, AbbVie, BMS, UCB, Crescendo Bioscience, Lilley, Pfizer, Roche,
5;
A. Williams,
Medimmune,
1,
Medimmune,
3;
N. Karlsson,
AstraZeneca,
1,
AstraZeneca,
3;
A. Godwood,
AstraZeneca,
1,
Medimmune,
3;
D. Close,
Medimmune,
3.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/analysis-of-patient-reported-outcomes-during-treatment-with-mavrilimumab-a-human-monoclonal-antibody-targeting-gm-csfra-in-the-randomized-phase-2b-earth-explorer-1-study/