Session Information
Date: Sunday, November 13, 2016
Title: Rheumatoid Arthritis – Small Molecules, Biologics and Gene Therapy - Poster I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: In previous clinical studies the human monoclonal TNFα-antibody golimumab (GLM) showed a good clinical response and a favorable benefit:risk profile in the treatment of moderate to severe active rheumatoid arthritis (RA). This was also shown in the clinical practice of the GO-MORE-study*. Until today it has not been examined whether the gathered GO-MORE study results can be referred to the clinical practice in Germany. The scope of the German subpopulation (GER) analysis was to investigate which are the treatment modalities of GLM in combination with predominately used methotrexate (MTX) and the different doses as well as other DMARD-combinations (e.g. leflunomide, LEF).
Methods: GO-MORE was a large open-label, multinational, prospective study in biologic-naïve patients (pts) with active RA*. The GER (n=370) was analysed in the context of the total study population (TSP, n=3.280; including GER) – purely descriptively as no formal comparison was performed. Good/moderate EULAR-(DAS28-ESR)-response depending on different DMARD-combinations and quality of life were evaluated. Enrolled pts (DAS28-ESR ≥3.2 despite DMARD-therapy) received 50mg GLM SC once monthly for 6 months.
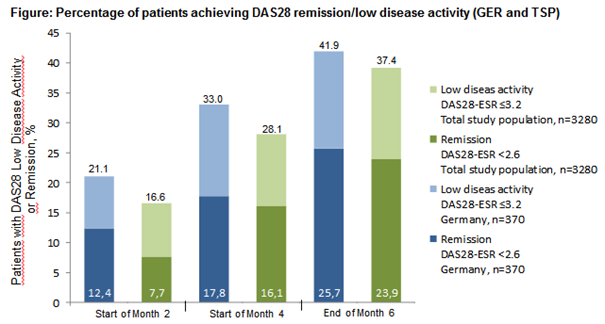
Results: Within both groups females were more often affected than males (82.8% in TSP; 74.1% in GER). After 6 months of treatment 80.3% (297/370) of the pts of the GER achieved good/moderate EULAR-(DAS28-ESR)-response (TSP 82.1% (2.692/3.280)). 70.5% received GLM as add-on-therapy to MTX-treatment (81.2% TSP). The pts were preferably treated with GLM in combination with only MTX (207/368, 56.3%). At 6 months good/moderate EULAR-(DAS28-ESR)-response was achieved by 81.2% of the GLM+MTX treated pts (GLM+LEF (66/368, 17.9%): 81.8%; GLM+MTX+LEF (34/368, 9.2%): 73.5%) and furthermore 25.7% of the GER pts got into remission. GLM was well tolerated.
MTX 15mg/week was most frequently used (29.7% compared to >15mg MTX/week: 22.2% and <15mg MTX/week: 18.7%). The respective EULAR-good/moderate-response rates at 6 months were: 82.7% (110/368), 78% (82/368) and 78.3 % (69/368). Moreover the good clinical response rates were concordant with the improvement of functionality and the quality of life. At 6 months 31.1% of the pts showed no/minimal functional impairment (HAQ-DI≤0.5) and the EQ-5D increased from 0.49 to 0.67 (37%).
Conclusion: German pts showed continuous clinical improvement rates over 6 months when treated with GLM, regardless to the chosen concomitant LEF/MTX-therapy. The collected data concerning EULAR-(DAS28-ESR)-response, quality of life and safety profile were comparable to those of the multinational GO-MORE-study. This validates the applicability of the data from the multinational GO-MORE-study to German clinical practice. Reference: * Combe B, et al. Ann Rheum Dis 73:1477–1486
To cite this abstract in AMA style:
Schulze-Koops H, Wollenhaupt J, Winnemöller M, Klaudius I, Löffler H. Analysis of a German Subpopulation with Active Rheumatoid Arthritis Treated with Golimumab As Add-on Therapy to Disease-Modifying Antirheumatic Drugs [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/analysis-of-a-german-subpopulation-with-active-rheumatoid-arthritis-treated-with-golimumab-as-add-on-therapy-to-disease-modifying-antirheumatic-drugs/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/analysis-of-a-german-subpopulation-with-active-rheumatoid-arthritis-treated-with-golimumab-as-add-on-therapy-to-disease-modifying-antirheumatic-drugs/