Session Information
Date: Wednesday, November 13, 2019
Title: 6W020: Miscellaneous Rheumatic & Inflammatory Disease III: Novel Therapies (2900–2905)
Session Type: ACR Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: The main goal of Familial Mediterranean Fever (FMF) treatment is to prevent attacks and subclinical inflammation. Mainstay of treatment is colchicine; however, 5-10% of patients are unresponsive to colchicine even in maximum tolerated dosage. Anakinra has been proven to be effective in controlled trials in terms of attack frequency and subclinical inflammation in colchicine resistant patients. The objective of this study is to review the patients followed in our single center with FMF who received Anakinra because of insufficient colchicine response.
Methods: The study is conducted in a tertiary rheumatology center experienced in autoinflammatory diseases. The patients are treated with at least one month of Anakinra in order to be included in the cohort. Patients with amyloidosis and pregnancy are not included. Genetic mutation of all patients was checked and recorded. Attack frequency, patient global assessment scales of disease severity (10-cm Visual Analogue Scale [VAS]), and acute phase reactants were recorded before and throughout Anakinra treatment. Treatment termination was also noted. Criteria of treatment termination were side effects, disease remission, inadequate response, pregnancy plan, and noncompliance.
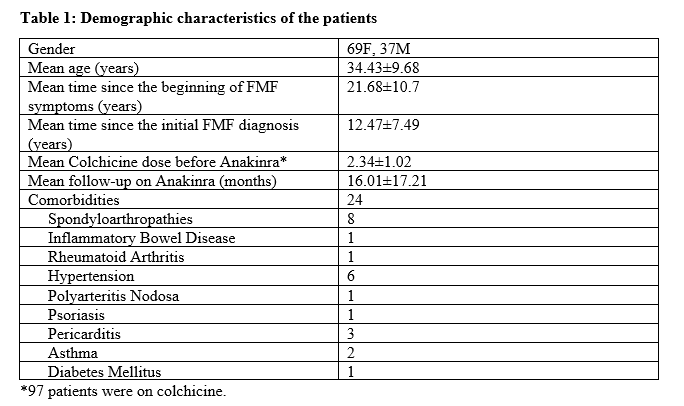
Results: 106 patients diagnosed with FMF were treated with Anakinra. Table 1 represents the demographic characteristics and FMF-related clinical features of the patients. 45.92% of our patients had a homozygous M694V mutation. 83 of the 98 patients tested for MEFV carried at least one copy of M694V. Attack frequency decreased while on Anakinra treatment. Seventy five patients reported no attacks after anakinra treatment whereas 16 patients reported at least 50% decrease in the attack frequency. VAS score decreased from 7.49±2.03 to 3.08±2.82 (p< 0.0001). Currently, 70 patients are still on Anakinra treatment. Treatment of 34 patients was discontinued (32% of all patients). The reasons of Anakinra treatment discontinuation are shown in table 2. Insufficient response and side effects are the most common reasons for treatment discontinuation. All of the side effects observed were reversible and the patients alleviated after treatment cessation. In 4 patients, leukopenia was observed and was managed with dose reduction to alternating treatment. Treatment of 8 patients was successful enough that it was terminated due to remission.
Conclusion: In conclusion, in patients with colchicine treatment failure, anti-IL-1 agent Anakinra is shown to be effective and safe. The effectiveness of Anakinra stems from preventing attacks, and increasing the quality of life.
To cite this abstract in AMA style:
Ugurlu S, Egeli B, Ergezen B, Selvi O, Ozdogan H. Anakinra Treatment in Patients with Familial Mediterranean Fever: A Single-center Experience [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/anakinra-treatment-in-patients-with-familial-mediterranean-fever-a-single-center-experience-2/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/anakinra-treatment-in-patients-with-familial-mediterranean-fever-a-single-center-experience-2/