Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Systemic lupus erythematosus (SLE) is characterized by pathogenic autoreactive B cells producing autoantibodies against multiple self-antigens. Recently, a series of clinical cases suggested that traditionally manufactured anti-CD19 chimeric antigen receptor T cell (CAR-T cell) therapies show potential to promote full clinical remission in severe refractory SLE (srSLE).1,2 This is the first clinical trial to evaluate the preliminary safety and efficacy of anti-CD19 CAR-T cell therapy in patients with srSLE. YTB323 is a novel, rapidly manufactured, autologous CAR-T cell therapy that has shown preserved T cell stemness and enhanced CAR-T cell efficacy in hematological malignancies.3
Methods: An open-label, single-arm, multicenter phase 1/2 study, CYTB323G12101 (NCT05798117), to assess the safety, efficacy and cellular kinetics of YTB323 in participants with srSLE is currently ongoing. A sentinel cohort of patients with srSLE (n=3), dosed with 12.5×106 cells at least 28 days apart, was enrolled. The primary endpoint was safety as measured by vital signs, adverse events, laboratory parameters and ECG evaluation. CAR-T cell kinetics were monitored by quantitative PCR and flow cytometry. Further relevant endpoints included changes from baseline levels in circulating B and T cells, autoantibody and complement levels, disease activity scores and renal outcome measures.
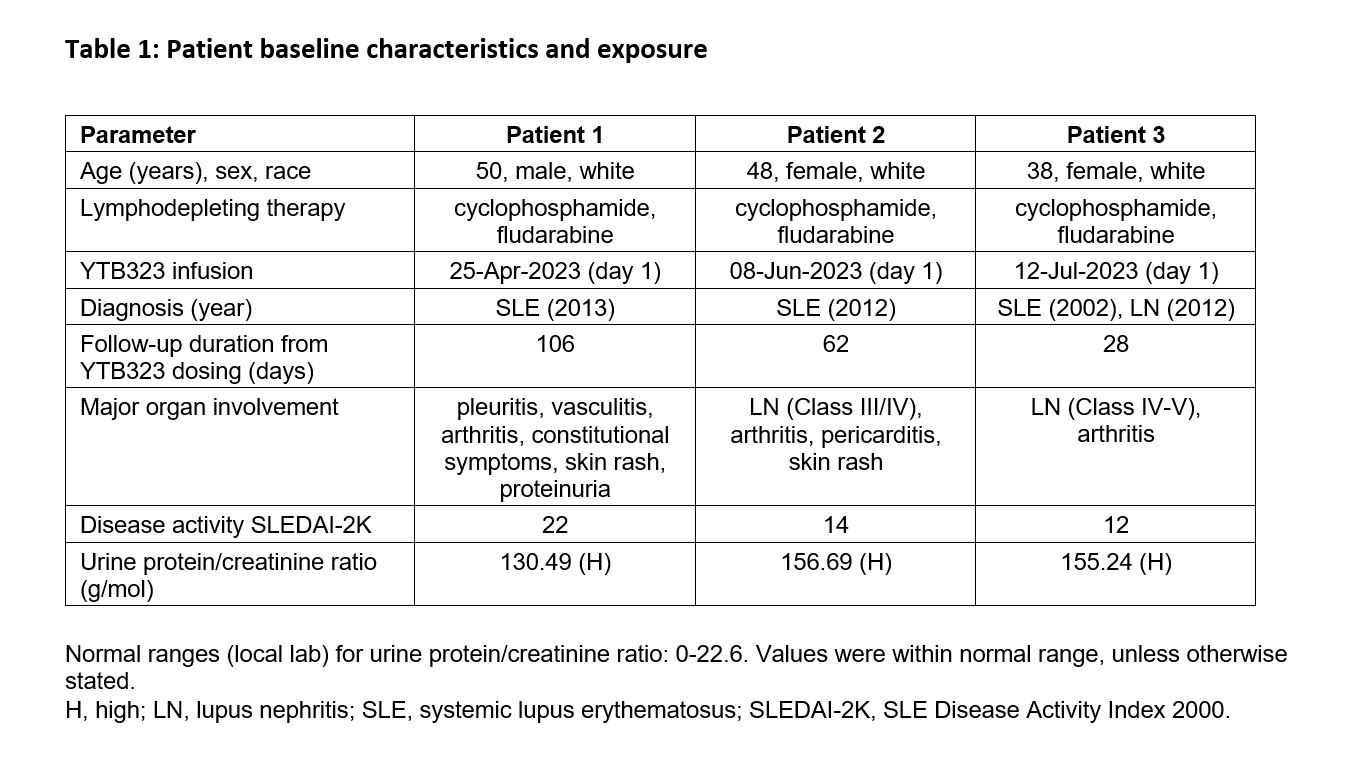
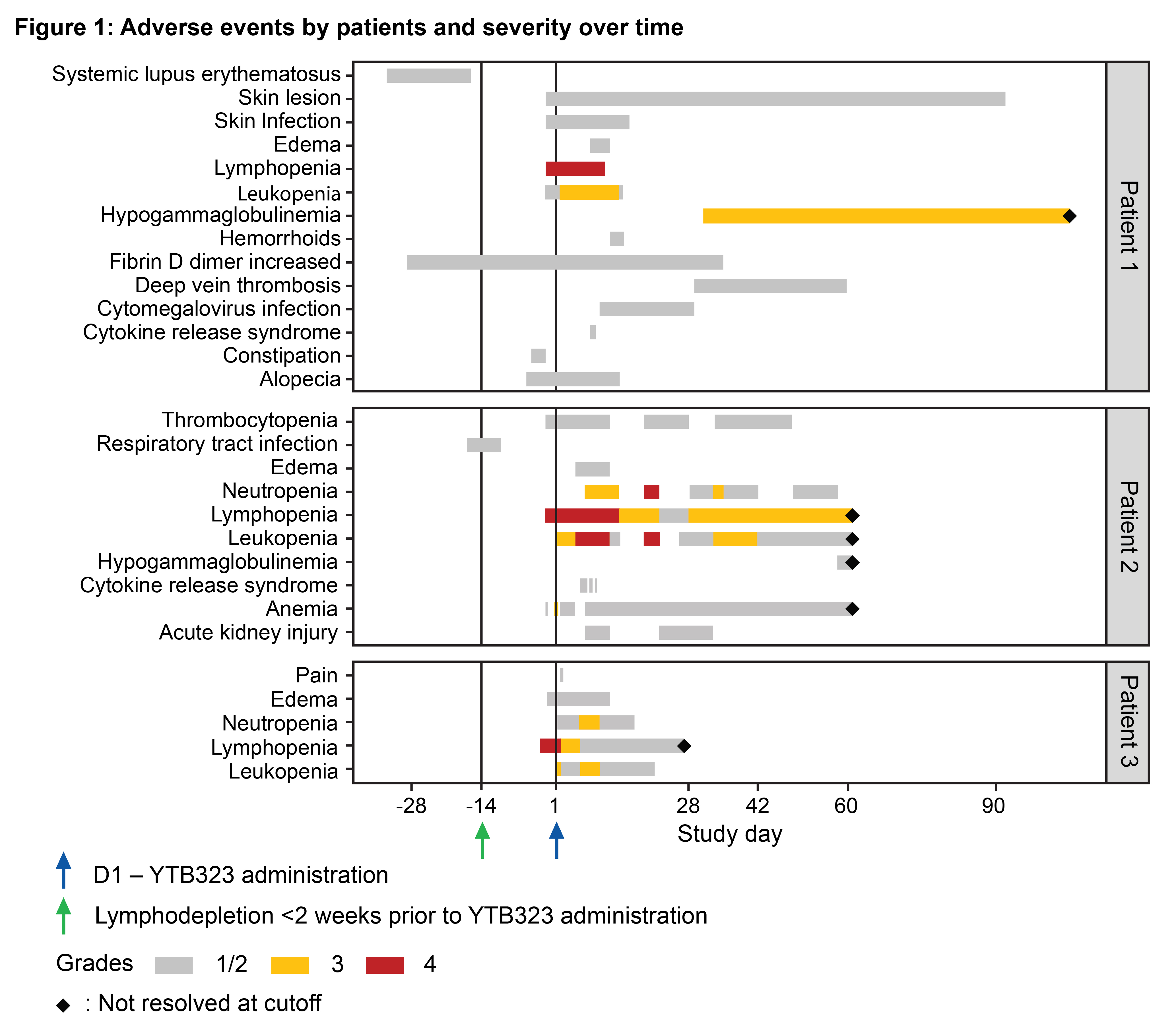
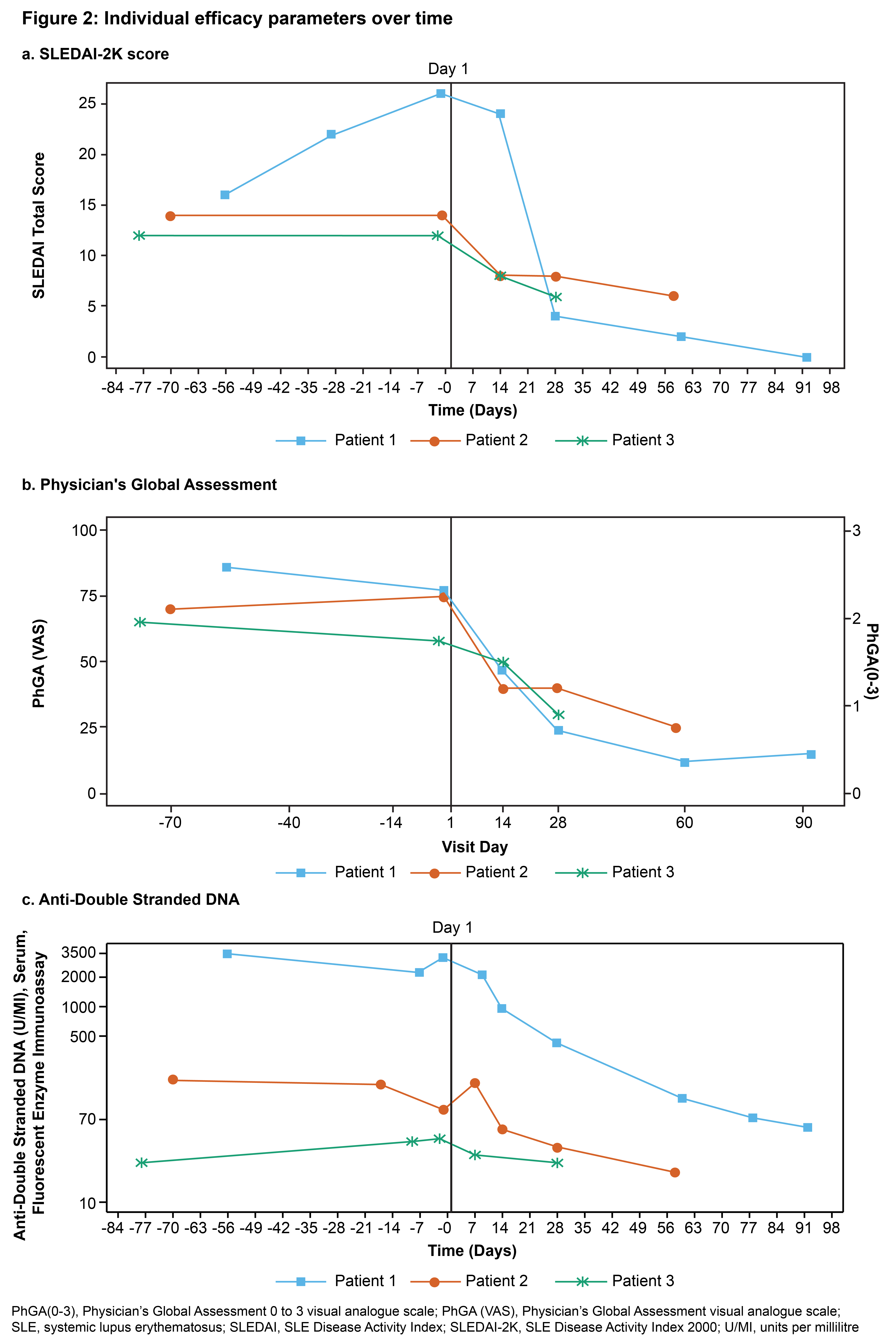
Results: Baseline characteristics of patients are summarized in Table 1. Adverse events are shown in Figure 1. No serious adverse events or deaths were reported. Following screening, the first patient experienced a worsening of disease during immunosuppressant washout prior to receiving lymphodepletion therapy. No immune effector cell-associated neurotoxicity syndrome (ICANS) occurred. A grade 1 cytomegalovirus reactivation occurred in one patient, which was subsequently resolved. Two patients had cytokine release syndrome (grade 1 or 2); both responded well to tocilizumab treatment and fully recovered. As expected, grade 3 and 4 transient lymphodepletion-related cytopenias were observed in all patients. Grade 2 or 3 hypogammaglobulinemia was observed in two patients; neither event required intravenous immunoglobulin treatment. Cellular kinetics studies showed peak expansion (Tmax) at 13 and 18 days post-infusion for the two patients with available data characterizing the expansion phase. Transient T cell and sustained B cell depletion were observed in all patients. Preliminary efficacy data (Figure 2) suggest substantial decreases in SLE Disease Activity Index (SLEDAI) (Figure 2a) and Physician’s Global Assessment (PhGA) (Figure 2b), in line with improvements in relevant disease biomarkers such as dsDNA (Figure 2c), complement levels and proteinuria.
Conclusion: Preliminary data from this clinical trial including the first three sentinel patients suggest favorable safety, CAR-T cell expansion, B cell depletion and initial efficacy, supporting continuation of the study to evaluate YTB323 in srSLE.
References
1. Mackensen et al. Nat Med (2022) 28:2124-32.
2. Schett et al. The EULAR Journal (2023) 93.
3. Dickinson et al. Cancer Discov (2023) 13:1982-1997.
To cite this abstract in AMA style:
Cortés Hernández J, Barba P, Linares Alberich M, Fischer O, Kovacs B, Calzascia T, Pearson D, Jordán Garrote A, Kirsilä T, Siegel R, Shisha T, Cavalli G, Gergely P. An Open-label, Multicenter, Phase 1/2 Study to Assess Safety, Efficacy and Cellular Kinetics of YTB323, a Rapid Manufacturing CAR-T Cell Therapy Targeting CD19 on B Cells, for Severe Refractory Systemic Lupus Erythematosus: Preliminary Results [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/an-open-label-multicenter-phase-1-2-study-to-assess-safety-efficacy-and-cellular-kinetics-of-ytb323-a-rapid-manufacturing-car-t-cell-therapy-targeting-cd19-on-b-cells-for-severe-refractory-system/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/an-open-label-multicenter-phase-1-2-study-to-assess-safety-efficacy-and-cellular-kinetics-of-ytb323-a-rapid-manufacturing-car-t-cell-therapy-targeting-cd19-on-b-cells-for-severe-refractory-system/