Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Objective:Therapeutic decisions are based on efficacy, but clinicians need to consider medication safety in this process. Here, we report ustekinumab (UST) integrated safety data in patients (pts) with psoriatic arthritis (PsA), Crohn’s disease (CD), and psoriasis (PsO). We also compare a subset of PsA pts with & without baseline methotrexate (MTX).
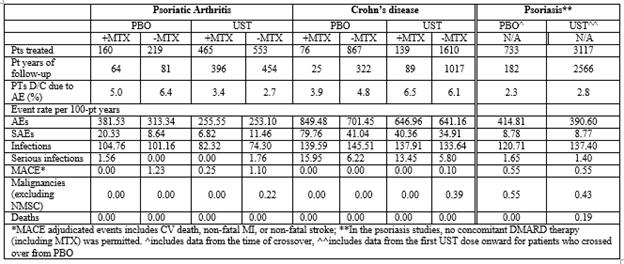
Methods: Integrated safety data from 3 PsA, 5 CD, & 4 PsO trials were analyzed. PsA studies included the Ph2 trial [CNTO743T10] & the 2 Ph3 trials (PSUMMIT 1 & 2) with 222, 615 & 312 pts exposed to UST, respectively. The percentage of pts in the Ph2 study that received MTX was 20.5%. No concomitant DMARDs with the exception of MTX (approximately 50% of pts in each study) were permitted in the 2 Ph3 studies. In the 5 CD trials (Ph2/3Ph3) 1749 pts were exposed to UST. In the 3 Ph3 CD trials, pts received one dose of UST 130mg or ˜6mg/kg IV at induction (UNITI-1 & UNITI-2), then 8 weeks later, entered the maintenance phase (IM UNITI) and received UST 90mg SC q8w or q12w for 44 weeks. The percentage of pts on background MTX in UNITI-1 was 9.2% & in UNITI-2, 4.8%. In the PsO studies (1Ph2/3Ph3), a total of 3117 pts received UST 45mg or 90mg SC; no concomitant DMARD therapy (including MTX) was permitted. The PsO studies were completed through 5-years of follow-up. All pts who received at least 1 dose of UST are included in this analysis. Safety events are reported in events per 100-pt years. 95% CI for events per 100 PY were estimated.
Results: Through 1 year of follow-up, a total of 1018 PsA pts were treated with UST, of which 465 were co-treated with MTX. Of the 1749 CD pts treated with UST, 139 received MTX. Discontinuation rates of UST due to adverse events (AEs) were comparable across disease states irrespective of MTX use (Table). AE rates (95% CIs for events/100 PY) were noted to be significantly lower in the UST vs PBO groups across the 3 diseases irrespective of MTX use-UST (420.39, 423.45) vs PBO (534.80, 570.44). Serious adverse event rates (SAEs) were also significantly lower in the UST vs PBO across the 3 diseases-UST (14.25, 16.56) vs PBO (24.05, 32.18). Infections and Serious infections (SIs) had numerically lower event rates in UST vs. PBO across the 3 disease states-UST (122.16, 128.72) vs PBO (120.94, 138.27) and UST (2.10, 3.05) vs PBO (2.76, 6.00), respectively (Table). Major adverse cardiovascular events (MACE) did not appear to significantly differ in both PBO & UST pts in PsA, PsO and CD. Event rates of malignancy (excluding non-melanoma skin cancer) were comparable across all disease states. No deaths in PsA or CD were reported.
Conclusion: UST demonstrates a favorable safety profile in an integrated safety data analysis across the PsA, CD, & PsO phase 2 & 3 clinical trials. The use of UST in PsA appears to be safe & well-tolerated, with fewer event rates of SAEs & SIs noted vs PBO. Despite higher overall rates of SAEs & SIs observed in CD pts, the data do not suggest an influence of UST on either.
To cite this abstract in AMA style:
Gensler LS, Hsia EC, Gasink C, Randazzo B, Parenti D, Fakharzadeh S, Tang KL, Chakravarty S. An Integrated Safety Data Analysis Across All Phase II and Phase III Clinical Programs for Ustekinumab in Psoriatic Arthritis, Crohn’s Disease, and Psoriasis [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/an-integrated-safety-data-analysis-across-all-phase-ii-and-phase-iii-clinical-programs-for-ustekinumab-in-psoriatic-arthritis-crohns-disease-and-psoriasis/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/an-integrated-safety-data-analysis-across-all-phase-ii-and-phase-iii-clinical-programs-for-ustekinumab-in-psoriatic-arthritis-crohns-disease-and-psoriasis/