Session Information
Date: Monday, November 6, 2017
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
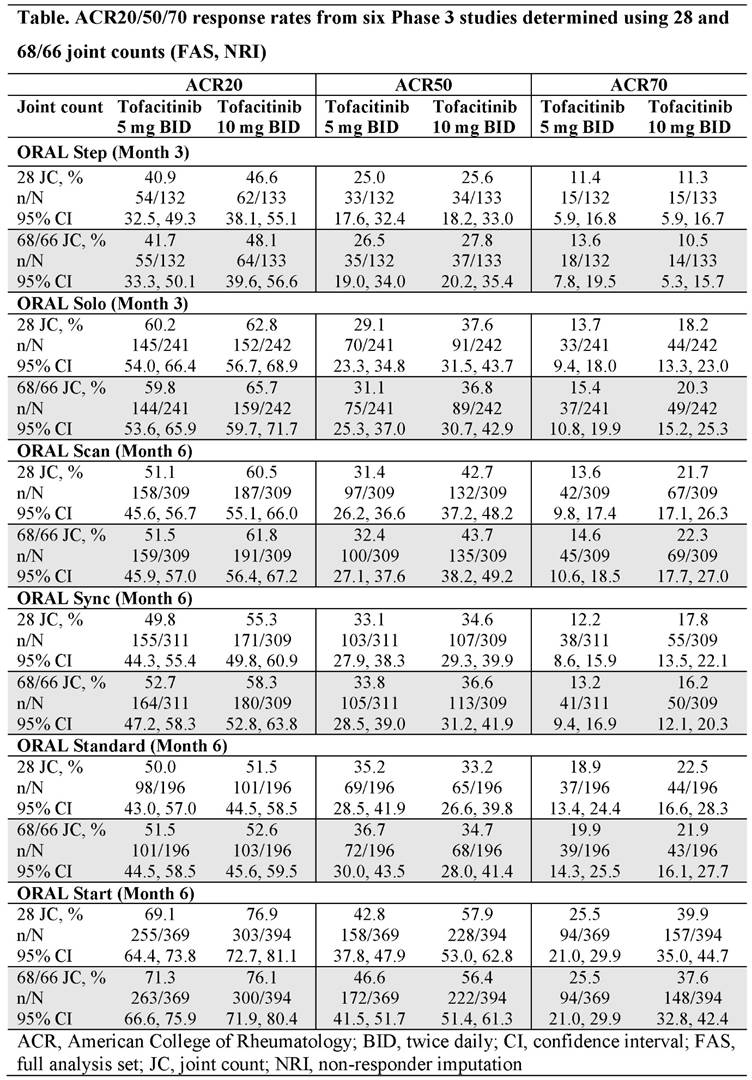
Background/Purpose: Tofacitinib is an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis (RA). In a clinical trial setting, standard criteria for measuring the effectiveness of treatments in patients with RA include American College of Rheumatology (ACR) response rates (defined as the proportion of patients with an improvement of 20/50/70%, respectively, in tender and swollen joint counts and 20/50/70% improvement in 3 of the 5 core components [patient and physician global assessments, pain, disability, and an acute phase reactant] between time points).1 Tender and swollen joint counts are typically assessed by counting 68 and 66 joints, respectively.2 However, the use of a 28 joint count is considered a valid and reliable way to determine ACR response rates,3 and a 28 joint count has been used to determine ACR response rates in the recently completed ORAL Strategy (NCT02187055) Phase 3b/4 study of tofacitinib. The current post-hoc analysis was conducted to determine whether ACR response rates in six Phase 3 studies of tofacitinib varied when a 28 joint count vs a 68/66 joint count was used.
Methods: Data from patients with active RA who received tofacitinib 5 or 10 mg twice daily (BID) in six randomized, double-blind Phase 3 studies (ORAL Step [NCT00960440], ORAL Solo [NCT00814307]; ORAL Scan [NCT00847613], ORAL Sync [NCT00856544], ORAL Standard [NCT00853385], and ORAL Start [NCT01039688]) were included in this analysis. ACR20/50/70 response rates were determined using 28 joint counts and compared with ACR20/50/70 response rates obtained in the respective study using the full joint count (68/66 joints). ACR response rates were assessed at the time of the primary endpoints of the studies (Month 3 in ORAL Step and ORAL Solo; Month 6 in all other studies). All analyses were based on the full analysis set; missing data were imputed using non-responder imputation.
Results: Overall, the ACR20/50/70 response rates of 3141 tofacitinib-treated patients were determined; 1558 (49.6%) and 1583 (50.4%) patients were randomized to receive tofacitinib 5 and 10 mg BID, respectively. At the primary endpoint, ACR20/50/70 response rates measured using the 28 joint count were generally similar to those determined using the full joint count within each study (Table). ACR20/50/70 response rates of placebo-treated patients were also generally similar when measured using the 28 and 68/66 joint counts (data not shown).
Conclusion: The results of this post-hoc analysis suggest that the use of a 28 joint count in tofacitinib-treated patients with RA provides similar ACR response rates as the use of a full joint count (68/66 joint count).
References:
1. Felson DT et al. Arthritis Rheum 1995; 38: 727-735.
2. Felson DT et al. Arthritis Rheum 1993; 36: 729-740.
3. ACR Committee on Outcome Measures in Rheumatoid Arthritis Clinical Trials. Arthritis Rheum 1994; 37: 463-464.
To cite this abstract in AMA style:
Smolen JS, Shergy W, Wright GC, DeMasi R, Kwok K, Mojcik CF, Iikuni N, Tatulych S, Citera G. American College of Rheumatology Response Rates Determined Using 28 Versus 68/66 Joint Count in Patients with Rheumatoid Arthritis Receiving Tofacitinib in Phase 3 Studies [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/american-college-of-rheumatology-response-rates-determined-using-28-versus-6866-joint-count-in-patients-with-rheumatoid-arthritis-receiving-tofacitinib-in-phase-3-studies/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/american-college-of-rheumatology-response-rates-determined-using-28-versus-6866-joint-count-in-patients-with-rheumatoid-arthritis-receiving-tofacitinib-in-phase-3-studies/