Session Information
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: While the pathogenesis of neuropsychiatric SLE (NPSLE) is not fully understood, critical aspects of disease development include neuroinflammation and loss of brain barrier integrity. Fingolimod, a sphingosine-1-phosphate (S1P) receptor modulator, is approved for the treatment of multiple sclerosis. Through functional antagonism of S1P receptors, fingolimod prevents lymphocyte egress from lymphoid organs. In addition to reducing CNS infiltration by peripheral lymphocytes, fingolimod also exhibits direct neuroprotective effects such as preserving brain barrier integrity and decreasing pro-inflammatory cytokine secretion by astrocytes and microglia. Given these effects, we hypothesized that fingolimod would attenuate neurobehavioral deficits in MRL-lpr/lpr mice, a validated NPSLE model.
Methods: Ten-week-old female MRL-lpr/lpr mice were treated thrice weekly with fingolimod (3 mg/kg) or vehicle alone (n=16/group) by intraperitoneal injection. After 4 weeks of treatment, mice were assessed for alterations in cognitive function or emotionality. The object placement and object recognition tests were performed to assess visuospatial and recognition memory. The swim test was used to assess despair-like immobility. Brains were paraffin-embedded for histological analysis and immunohistochemical staining.
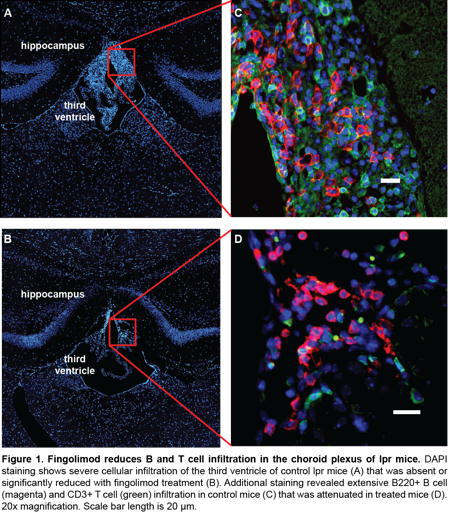
Results: Fingolimod-treated mice exhibited significantly improved preference for novel object placement when compared with control-treated mice (67±5% vs. 51±4.8%, p=0.024), demonstrating maintenance of visuospatial memory. Moreover, fingolimod-treated mice displayed less despair-induced immobility (57±2.7% vs. 45±4.2%, p=0.019), indicating that fingolimod mitigates the depression-like phenotype. Immunofluorescent staining revealed a significant reduction in B and T cell infiltration of the choroid plexus in fingolimod-treated mice (Figure 1). Finally, fingolimod reduced perivascular and periventricular leakage of serum albumin, supporting improved barrier functionality. Systemic effects of fingolimod included a significant decrease in splenomegaly and lymphadenopathy, but surprisingly without a change in circulating anti-DNA titers.
Conclusion: Fingolimod treatment ameliorates cognitive dysfunction and depressive-like behavior in MRL-lpr/lpr mice, and reduces CNS lymphocytic infiltration and brain barrier leakiness. Our results highlight a novel role of S1P in the pathogenesis of NPSLE and point to S1P receptor modulation as a potential therapeutic target.
To cite this abstract in AMA style:
Mike E, Stock A, Putterman C. Amelioration of Neuropsychiatric Systemic Lupus Erythematosus By Fingolimod-Mediated Sphingosine-1-Phosphate Receptor Modulation [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/amelioration-of-neuropsychiatric-systemic-lupus-erythematosus-by-fingolimod-mediated-sphingosine-1-phosphate-receptor-modulation/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/amelioration-of-neuropsychiatric-systemic-lupus-erythematosus-by-fingolimod-mediated-sphingosine-1-phosphate-receptor-modulation/