Session Information
Date: Saturday, November 7, 2020
Title: SLE – Treatment Poster I
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Therapeutic blood monitoring is not yet an accepted practice in rheumatology. Although hydroxychloroquine (HCQ) blood level monitoring has been utilized in limited pharmacokinetic studies for some time, evidence for the utility of HCQ therapeutic blood monitoring in clinical practice has accumulated in recent years. Blood levels, rather than plasma levels, accurately reflect exposure and measure adherence over the last month. HCQ blood levels predict retinopathy (Arthritis Rheumatol 2020;72:448–53) but also predict benefit, such as prevention of thrombosis (Abstract OP0160, European e-Congress of Rheumatology 2020, June 3-7). We compared HCQ blood levels from the same visit done at two laboratories to ascertain agreement.
Methods: All patients met revised ACR or SLICC classification criteria for SLE. Of the 114 patients included, 15 patients had 1 visit, 27 2 visits, 71 3 visits, and 1 patient 4 visits for a total of 286 visits. Patients were 85% female, 37% African American, and 51% Caucasian. HCQ blood levels were measured using the method of Füzéry et al. (Clin Chim Acta 2013;421:79–84) with a coefficient of variation < 3%, and at Exagen Diagnostics using liquid chromatography coupled to mass spectrometry (AVISE® HCQ). The Bland-Altman plot was generated using R package BlandAltmanLe. Intra-class correlation coefficient (ICC) was calculated using irr package in R version 3.6.1. Deming regression was performed using mcr package in R. Confidence bounds (95%) were calculated with the bootstrap method.
Results: Figure 1 shows the comparison of the University HCQ blood levels to the commercial HCQ blood levels. The Bland Altman plot showed the difference between the two measures of HCQ blood level of each patient against the average of the two measures (Figure 2). The mean difference between the two methods was -13.9, 95% limits of agreement were (-529.3, 501.5), and 4.2% (n=12) data points fell outside of the confidence limits. Intra-class correlation coefficient was used to calculate an estimate of the overall agreement between the two measurements. The ICC was 0.923 (0.904, 0.938). Deming regression was used to look at systematic differences between the two HCQ blood level measurement methods (Figure 3). The slope of best fit was 1.12 with 95% confidence limits of (0.99, 1.22).
Conclusion: There is excellent agreement between a university and commercial laboratory in measuring HCQ blood levels. Clinicians can be confident in the reliability of the hydroxychloroquine blood level. This should increase acceptance and utilization. Hydroxychloroquine blood levels have proven there is a “therapeutic window” to maintain benefit and still reduce risk of retinopathy.
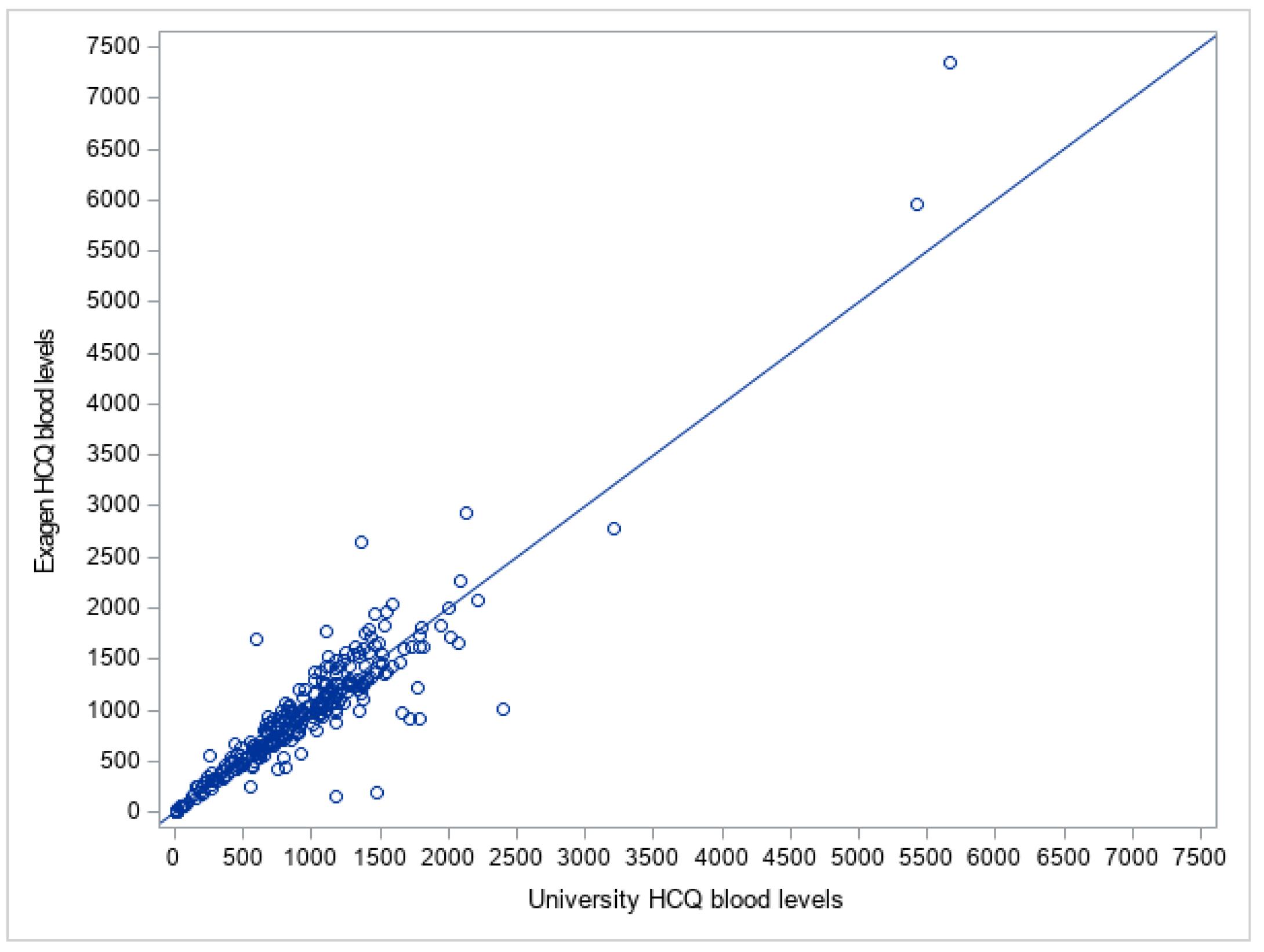 Comparison of the University Hydroxychloroquine Blood Levels to the Exagen Hydroxychloroquine Blood Levels
Comparison of the University Hydroxychloroquine Blood Levels to the Exagen Hydroxychloroquine Blood Levels
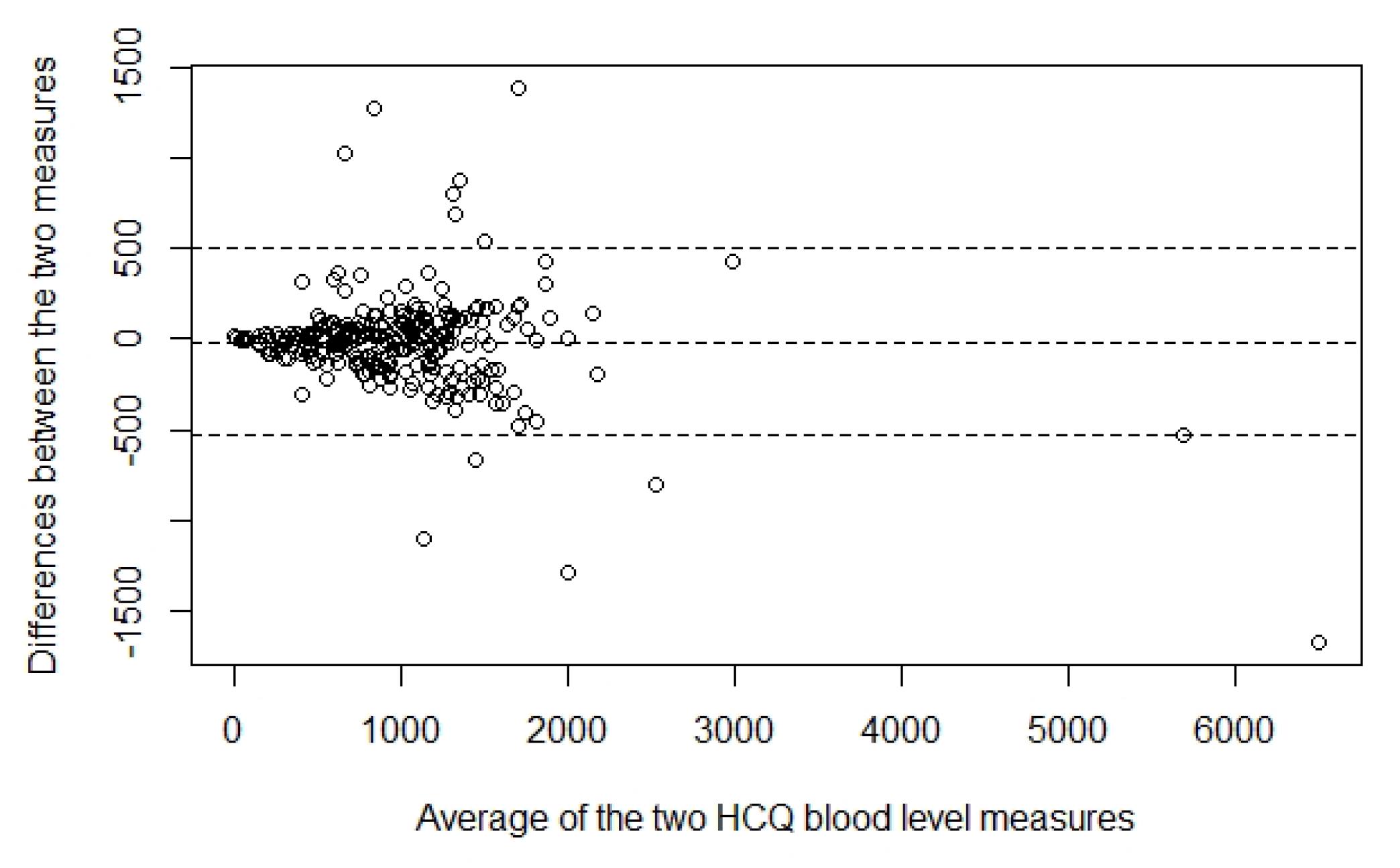 Bland-Altman Plot Showing Differences Between the Two Measures of Hydroxychloroquine Blood Level
Bland-Altman Plot Showing Differences Between the Two Measures of Hydroxychloroquine Blood Level
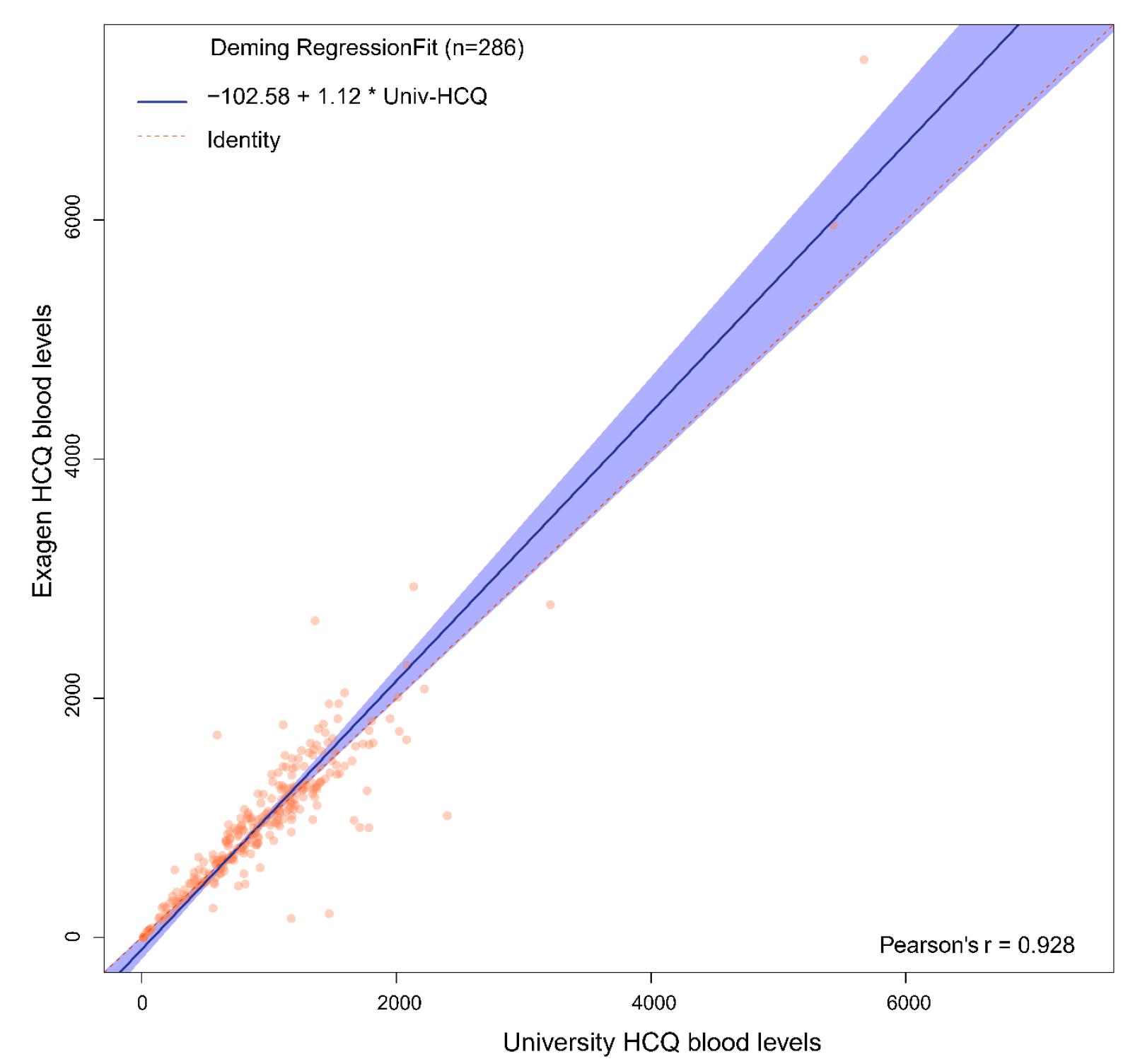 Deming Regression Plot Looking at Systematic Differences Between the Two Hydroxychloroquine Blood Level Measurement Methods
Deming Regression Plot Looking at Systematic Differences Between the Two Hydroxychloroquine Blood Level Measurement Methods
To cite this abstract in AMA style:
Petri M, Li J, Brady K, Conklin J, O'Malley T, Dervieux T. Agreement of Hydroxychloroquine Blood Levels Between a University and Commercial Laboratory [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/agreement-of-hydroxychloroquine-blood-levels-between-a-university-and-commercial-laboratory/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/agreement-of-hydroxychloroquine-blood-levels-between-a-university-and-commercial-laboratory/
