Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Administrative claims are often used to evaluate oral glucocorticoid (GC) use in RA for clinical and research purposes, despite limited evidence to support their accuracy. A prior study found a positive predictive value (PPV) of 65% for for GC use in Medicare claims compared to physician report in the CorEvitas registry (Galvao et al Pharm Drug Saf 2023). We are not aware of studies evaluating accuracy of GC claims vs. self-reported use.
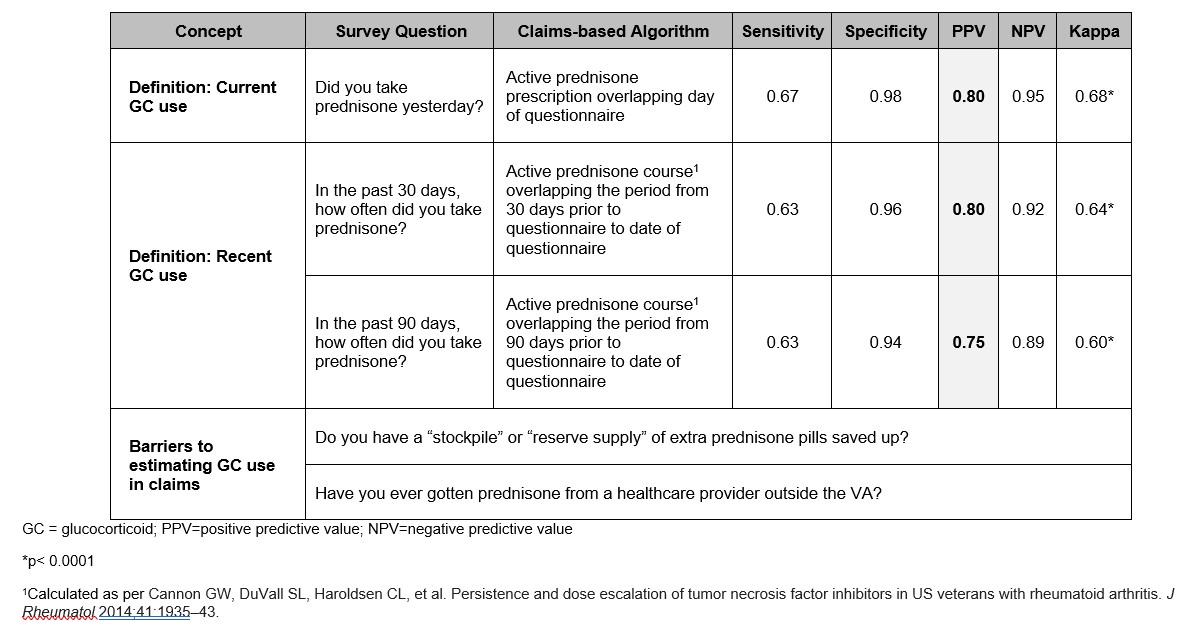
Methods: As part of a quality improvement initiative to improve GC use assessment in RA, patients at 7VA Rheumatoid Arthritis (VARA) registry sites filled out a 6-item questionnaire between Oct. 2023 and May 2024. Three items reflecting commonly used definitions of GC use (current use and recent use in the past 30 and 90 days, dichotomized yes/no) were used as reference standards to evaluate claims-based algorithms of prednisone dispensing from the VA Corporate Data Warehouse (CDW). We calculated sensitivity, specificity, PPV, and negative predictive value for each algorithm using the questionnaire item as a reference standard. As there is no validated benchmark for GC use, we calculated Cohen’s Kappa to assess agreement without a presumed reference standard. The questionnaire also assessed two potential barriers to accurate claims-based evaluation of GC use: “stockpiling” and obtaining GC from non-VA providers.
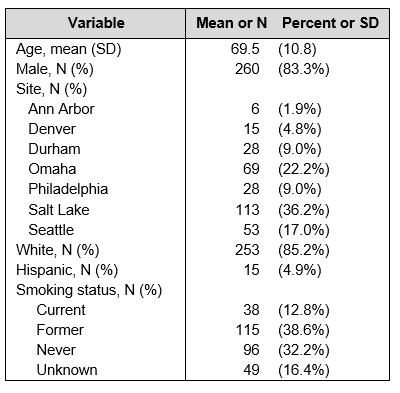
Results: Of 346 questionnaires, 312 (90.1%) were from unique patients and could be linked to CDW pharmacy claims. Of these, 36 (11.5%) reported current GC use, 56 (17.9%) reported use in the past 30 days, and 67 (21.5%) reported use in the past 90 days. Demographics reflect those of prior studies in this population (Table 1).
Table 2 shows the performance of each claims-based algorithm. The algorithm for current GC use had a sensitivity of 67%, specificity of 98%, and PPV of 80%. Algorithms for use in the past 30 and 90 days had sensitivities of 63% and 63%, specificities of 96% and 94%, and PPVs of 80% and 75%, respectively. Kappas were substantial and similar across algorithms (64-68%). Seventy respondents (22.4%) reported a GC stockpile. Eighty-nine respondents (28.5%) reported obtaining GC from a non-VA provider; 21 (6.7%) had done so in the past year.
Conclusion: Claims-based algorithms evaluating current and recent GC use performed acceptably compared to self-report, and better than prior assessments benchmarked on physician report. However, self-report of current GC use showed only 68% agreement with an active prednisone prescription. This suggests that a simple review of active medications by the physician is often inaccurate, even when assessing ongoing GC use. Agreement and PPV worsened as the interval being evaluated increased, which may reflect recall bias captured in the questionnaire vs. reduced accuracy in claims-based metrics applied to a longer assessment period. Over 1 in 5 patients reported having a GC stockpile, and 1 in 15 reported obtaining GC from outside VA in the past year, reflecting additional barriers to accurately estimating GC use via pharmacy claims.
To cite this abstract in AMA style:
Wallace B, England B, Baker J, brian S, Rojas J, Roul P, George M, Wysham K, Brubeck H, Smith I, Caplan L, Monach P, Kerr G, Kunkel G, Braaten T, Mikuls T, Cannon g. Agreement of Administrative Pharmacy Dispensing with Patient-Reported Use of Oral Glucocorticoids in a Rheumatoid Arthritis Cohort [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/agreement-of-administrative-pharmacy-dispensing-with-patient-reported-use-of-oral-glucocorticoids-in-a-rheumatoid-arthritis-cohort/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/agreement-of-administrative-pharmacy-dispensing-with-patient-reported-use-of-oral-glucocorticoids-in-a-rheumatoid-arthritis-cohort/