Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Colchicine is a widely used drug used to treat rheumatic and inflammatory conditions. Due to its long historical use in medicine, controlled clinical trials of colchicine have been small, precluding clear understanding about safety profile. The aim of the study was to systematically examine the adverse event (AE) profile of colchicine in randomized controlled trials (RCTs) across all published indications.
Methods: A systematic search was undertaken using electronic databases and manual searching of reference lists. The analysis included double-blind RCTs that compared the effects of oral colchicine to placebo or active comparator. Trials were included if they reported the incidence of AEs per group. AE data were extracted under pre-defined categories: diarrhoea, gastrointestinal events (including diarrhoea), liver events, hematology events, muscle events, sensory events, infection events and death, and any reported AE. Meta-analyses were undertaken to determine the pooled risk ratios (RR) of AEs in the colchicine group compared to the placebo and/or active comparator groups. Subgroup analyses were used to explore the effects of disease indication, dose, and exposure duration.
Results: Thirty-two studies were included involving participants with liver diseases (n = 6), gout (n = 5), Bechet’s and related conditions (n = 4), pericarditis and related conditions (n = 6), and other (n = 11). The pooled sample size was 3,774 participants. Any adverse event was reported in 26.6% of colchicine users compared to 20.9% of comparator groups, with an estimated risk ratio (RR) (95% confidence interval (CI)) of 1.72 (1.33-2.23) (Table). Sub-group meta-analyses showed no significant difference in RR of AEs in colchicine users between placebo and active comparator groups, or between different disease indications, duration of drug exposure, daily dose or cumulative dose. The RR (95% CI) in colchicine users compared to comparator groups for diarrhoea was 2.63 (1.67-4.16), and for any gastrointestinal AE was 1.97 (1.50-2.58), both P < 0.001. The RRs of liver, muscle (including myalgia, cramps, myotoxicity, and weakness), sensory, and infection AEs in colchicine users compared to comparators were not significant (Table). No study reported rhabdomyolysis, hematology AEs or deaths.
Conclusion: Although AEs are more common with colchicine compared with placebo or active comparator, these relate mostly to well-recognized gastrointestinal AEs. Increased incidence of liver, sensory, muscle, infection, or haematology AEs or death was not observed.
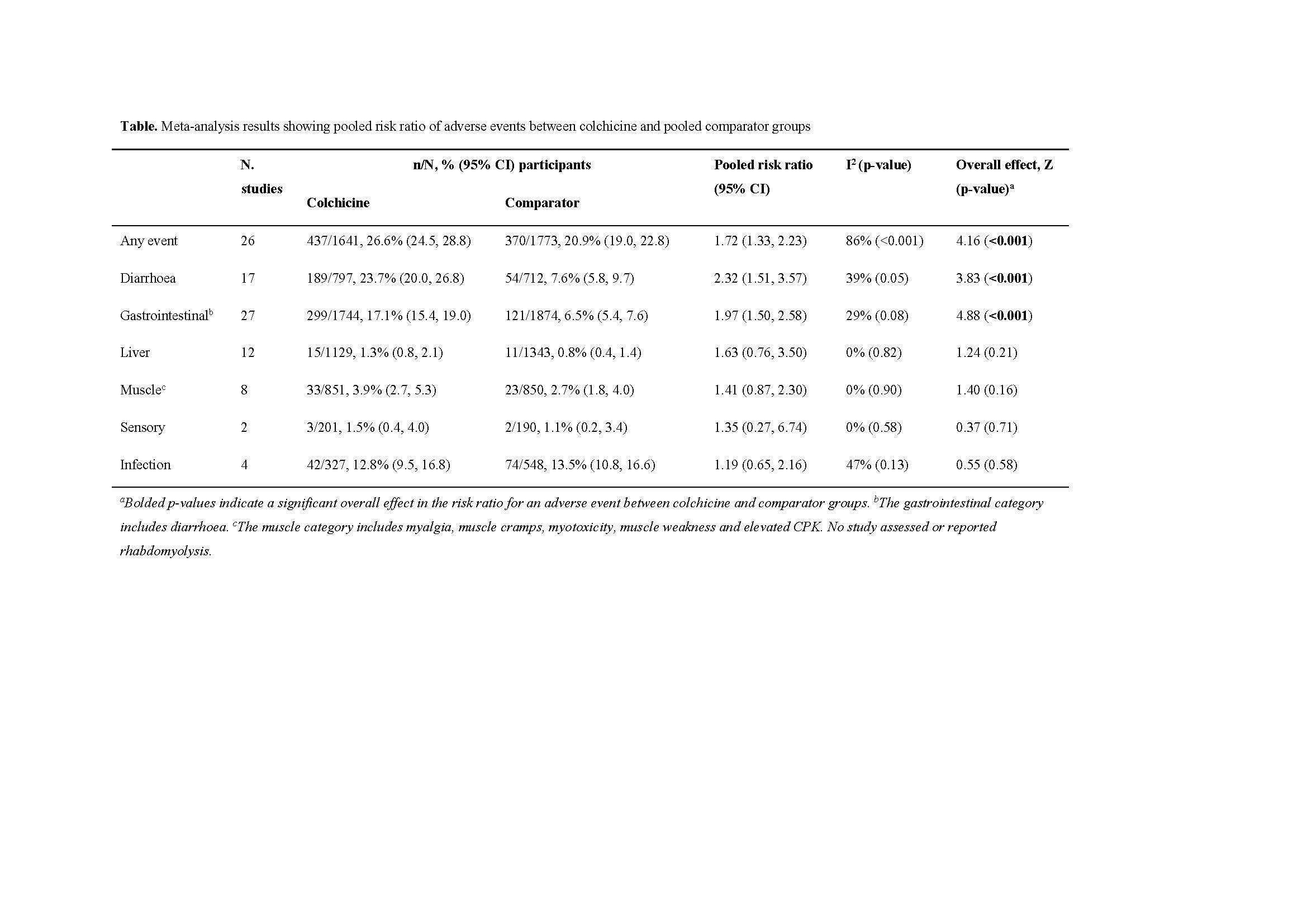
G-CAN colchicine abstract table
To cite this abstract in AMA style:
Stewart S, Yang K, Atkins K, Dalbeth N, Robinson P. Adverse Events During Colchicine Use: A Systematic Review and Meta-Analysis of Randomized Controlled Trial Events [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/adverse-events-during-colchicine-use-a-systematic-review-and-meta-analysis-of-randomized-controlled-trial-events/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/adverse-events-during-colchicine-use-a-systematic-review-and-meta-analysis-of-randomized-controlled-trial-events/
