Session Information
Date: Sunday, November 8, 2015
Title: Vasculitis I
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Currently-used
outcome measures in vasculitis insufficiently capture the life impact of
systemic vasculitis from patients’ perspectives. The Patient Reported Outcome
Measurement Information System (PROMIS) is a collection of item banks designed to
cover a broad range of self-reported health. This study assessed the
feasibility and construct validity of selected PROMIS instruments in a
longitudinal cohort of patients with vasculitis.
Methods: Data
from a multicenter longitudinal cohort of subjects with systemic vasculitis from
May 2014 to February 2015 were used. Instruments from 10 PROMIS item banks were
selected (Table 1) with direct involvement of patient research partners and
added to ongoing disease assessments. Each subject completed PROMIS instruments
from 6 item banks. PROMIS instruments were administered using computer adaptive
testing (CAT) intended to allow for a more precise estimate while minimizing
burden on study subjects. The Short Form 36 (SF-36) and physician and patient global
assessments for disease activity were also measured on an 11 point scale (0-10).
Active disease was defined as a physician global assessment >0. Cross-sectional
construct validity was assessed by calculating the correlations of PROMIS
scores with the other disease measures at baseline and longitudinal construct
validity was assessed by correlations of between-study visit differences in
PROMIS scores with differences in other disease measures.
Results: 604
study subjects came for 899 study visits. PROMIS assessments were completed at
796 (88%) of the visits. The median time to complete the set of PROMIS
assessments was 8.7 minutes (IQR 6.1-12.1) for the total cohort, 15.2 minutes
(IQR 11.9-19.2) for those older than 80, and 6.7 (IQR 4.9-9.6, )) minutes for
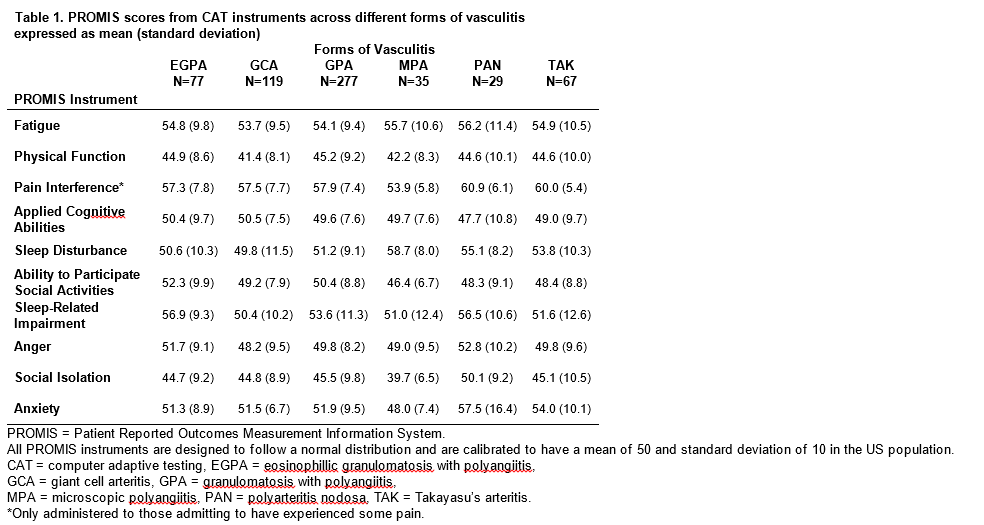
those younger than 40. Mean PROMIS scores at baseline are shown in Table 1. PROMIS
instruments correlated cross-sectionally with the individual scales of the
SF-36, most strongly with subscales of the SF-36 addressing the same domain as
the PROMIS instrument. Weaker correlations were observed in differences of
scores longitudinally. The differences in all PROMIS scores during active
disease vs. remission were in the expected direction for each domain (Table 2).
Conclusion:
PROMIS measures have cross-sectional construct validity and help discriminate
between active disease and remission. Inclusion of PROMIS instruments in disease
assessment in vasculitis would enhance capture of patients’ perspectives of disease
burden and complement traditional physician-based outcome measures.
To cite this abstract in AMA style:
Tomasson G, Farrar JT, Cuthbertson D, McAlear C, Asdown S, Gebhart D, Lanier G, Milman N, Peck J, Robson J, Carette S, Hoffman GS, Khalidi NA, Koening CL, Langford CA, Moreland LW, Monach PA, Pagnoux C, Specks U, Sreih AG, Ytterberg SR, Merkel PA. Administration of Patient Reported Outcome Measurement Information System (PROMIS) Instruments By Computer Adaptive Testing in Patients with Systemic Vasculitis [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/administration-of-patient-reported-outcome-measurement-information-system-promis-instruments-by-computer-adaptive-testing-in-patients-with-systemic-vasculitis/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/administration-of-patient-reported-outcome-measurement-information-system-promis-instruments-by-computer-adaptive-testing-in-patients-with-systemic-vasculitis/