Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
Conflicting data have been presented on patient compliance with methotrexate (MTX) prescription. A Danish study found a mean period of 12.3% of the prescribed years in reality being non-covered by MTX1, while recent data from Canada indicate that a rather large percentage of patients do not comply with the prescription of MTX2. The objective was to elucidate and quantify the use of MTX after a primary prescription for rheumatoid arthritis (RA).
Methods:
The study was a register based cohort study including patients with a hospital diagnosis extracted from the National Hospital Discharge Register of RA (ICD10 codes M05.X, M06.X), diagnosed after January 1, 1998 and aged ≥18 years at the date of first diagnosis/contact. The register has a nationwide coverage, and an almost 100% capture of contacts3. Data was extracted of all patients registered as MTX users in the Pharmacies Register from 1996 onwards. Any drugs bought are registered with an ATC code, number and dosage of product, form of medication (tablets, injections etc.), and date of sale. Stability of MTX use was defined as regular MTX prescriptions of 100 tablets of 2.5 mg delivered from a pharmacy > 1 per year with cut-off of 6, 9, or 12 months respectively corresponding to an average weekly dose of minimum 10; 7.5; and 5 mg respectively
These sources of information were linked through the Central Person Register Number which is a unique registration code given to every inhabitant – to some degree similar to the American social security number – that allows registration on an individual basis. The project was approved by the Danish National Data Protection Agency.
Results:
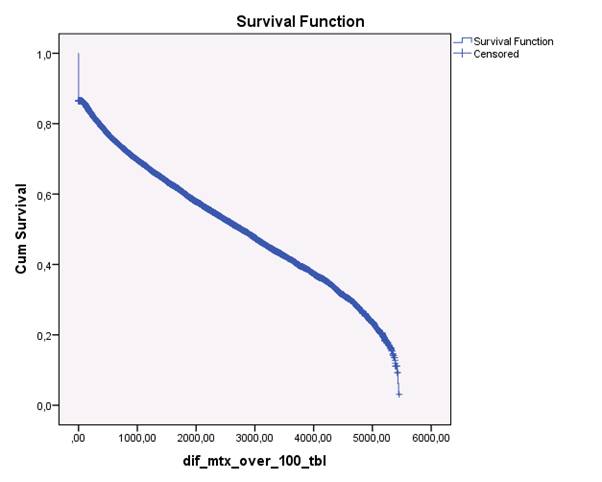
The study population consisted of 5,368,354 inhabitants in Denmark, among whom 4,191,428 were ≥ 18 years during the study period. 39,286 had the diagnosis of ICD10: M05 or M06 after January 1st 1998 and n= 18,703 were found to have used a prescription of MTX, representing 47.6 per cent of all subjects diagnosed with RA at a hospital in the period 1998-2011. Of these subjects, 2,200 (12 %) only used a prescription on MTX once, Figure 1. The median adherence in days almost doubled from the estimated dose of 10 mg to 5 mg per week: 5 mg: 5433 days, 7.5 mg: 4002 days, 10 mg: 2768 days.
Figure 1. Kaplan-Meyer plot of the remaining subjects on MTX therapy the period 1998-2012. In this plot, the assumption was that the subject had a prescription of > 1 per year, i.e. a minimum average dose of ≥10 mg per week. The last part of the curve, i.e. > 5,000 days is dipping, presumably due to lack of sufficient follow-up time after the last prescription.
Conclusion:
Adherence to MTX therapy was decreasing with increasing defined minimum doses 10; 7.5; and 5 mg weekly respectively. The median treatment time on these doses, however, was very long, e.g. 7.5 years on the 10 mg dose. Even so, the results indicate that patients taper their MTX over the years.
Disclosure:
H. Bliddal,
None;
R. Christensen,
Abbott, Axellus A/S, Bristol-Myers Squibb, Cambridge Weight Plan, Norpharma, Pfizer, and Roche,
5,
Axellus A/S, Cambridge Weight Plan, Mundipharma, and Roche,
2,
Abbott, Axellus, Bayer HealthCare Pharmaceuticals, Biogen Idec, Bristol-Myers Squibb, Cambridge Weight Plan, Ipsen, Laboratoires Expanscience, MSD, Mundipharma, Norpharma, Pfizer, Roche, and Wyeth,
8;
M. Østergaard,
None;
T. Lorenzen,
Phizer, Roche,
6;
M. S. Hansen,
None;
P. Vestergaard,
None;
S. A. Eriksen,
None.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/adherence-to-methotrexate-therapy-among-patients-with-rheumatoid-arthritis-in-denmark-a-registry-based-cohort-study/