Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: The laboratory diagnostics of antiphospholipid syndrome (APS) takes into account the persistent positivity for anticardiolipin (aCL) and/or anti-β2glycoprotein I (anti-β2GPI) antibodies and/or the presence of lupus anticoagulant (LA). However, testing for extra-criteria antiphospholipid antibodies (aPL) is emerging as a potential tool to improve diagnostic accuracy in patients suspected for APS. In this study we aimed to evaluate the clinical accuracy of aPL specificities both individually and/or in combination, in a wide cohort of patients suspected for APS [on the basis of the presence of thrombosis and/or pregnancy morbidity (PM-)] in an attempt to identify a panel of tests that may provide the best accuracy for diagnosing APS.
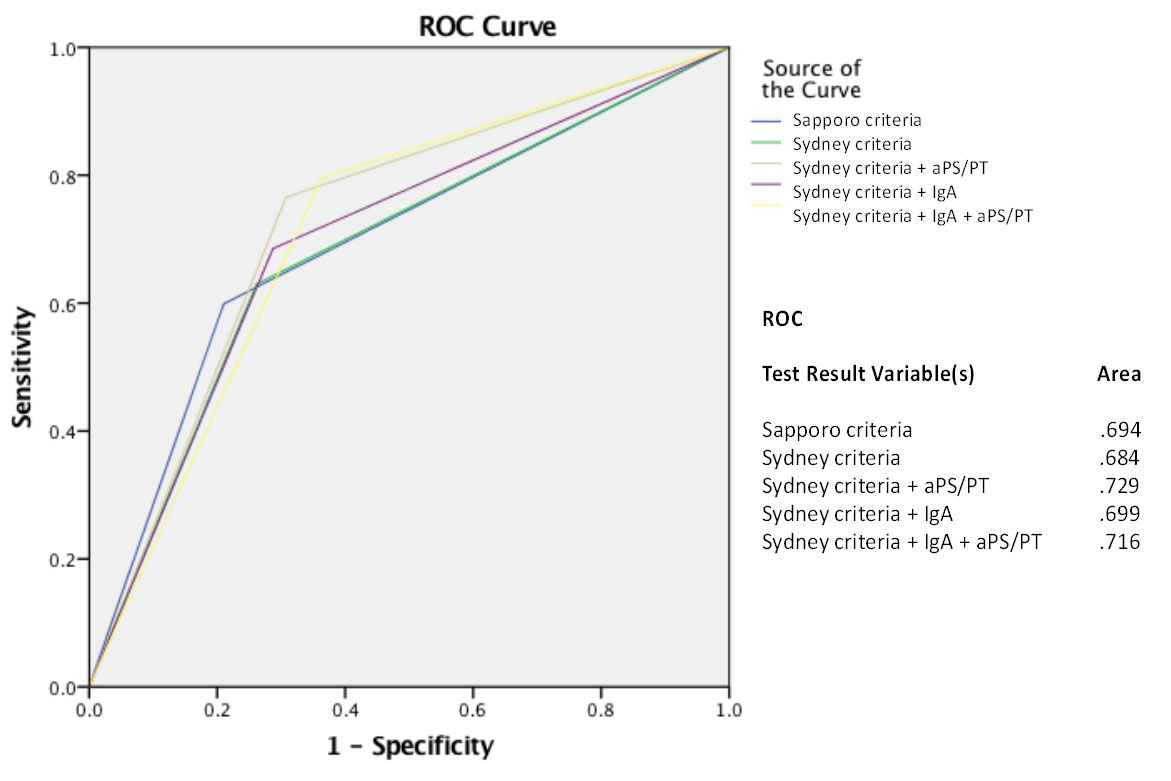
Methods: This study included 277 patients affected by an autoimmune systemic disease (234 women, mean age 44.7 ± 13.9 years). One hundred-forty-four patients suffered at least one thrombotic event or PM as described in the current classification criteria for APS. All patients were tested for LA, aCL, anti-β2GPI and anti-phosphatidylserine/prothrombin (aPS/PT). Testing for aCL, anti-β2GPI, and aPS/PT included IgG/M/A isotypes using a novel particle-based multi-analyte technology (PMAT, Aptiva System, Inova Diagnostics, research use only). Sensitivity, specificity, and predictive values were calculated. The diagnostic accuracy for each combination of tests was assessed by receiver operating characteristic (ROC) curve analysis and their area under the curve (AUC) was calculated.
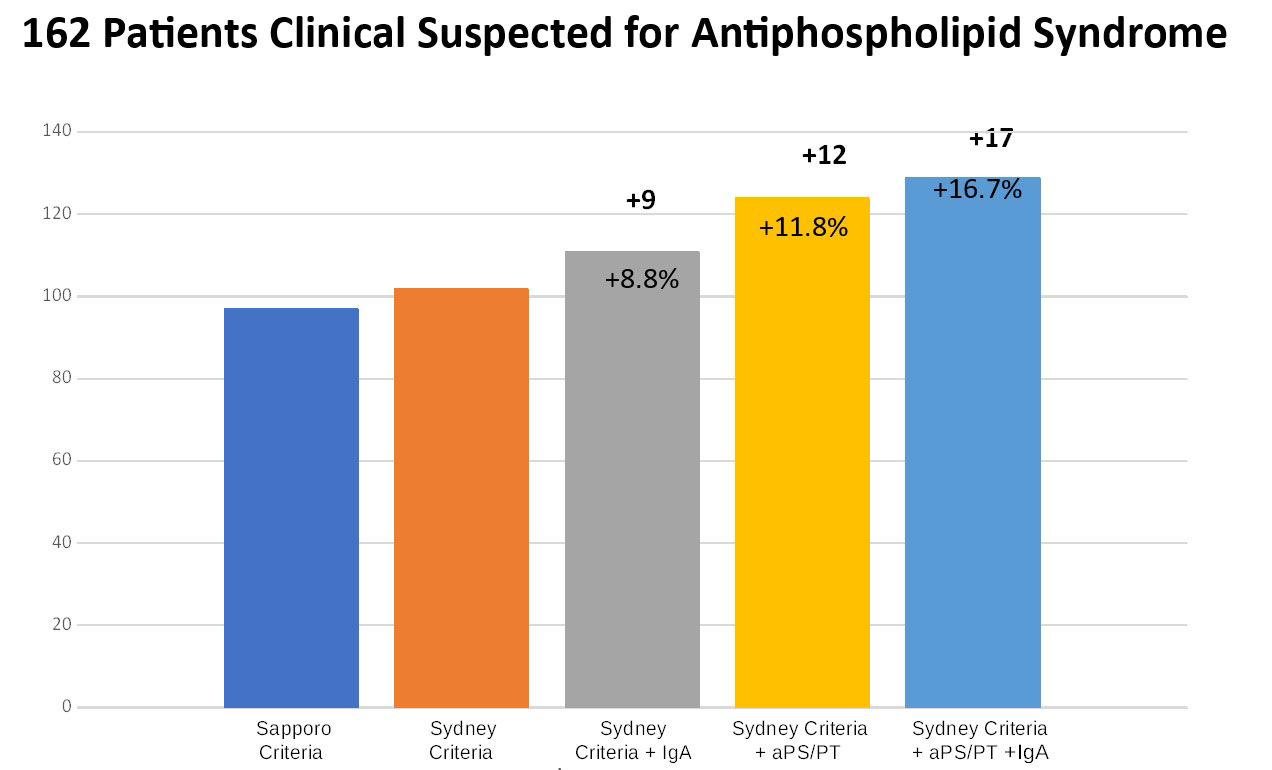
Results: Six possible combinations of results were derived from testing for 4 aPL (Figure 1). Among them, LA+/aCL+/aβ2GPI+/aPS/PT had the best diagnostic accuracy for APS [AUC 0.73, OR 4.0 (95% CI 1.9-6.0)]. Introducing testing for IgA and aPS/PT in the diagnostic workout of patients for suspected APS improved the diagnostic accuracy up to 16.7% (Figure 2).
Conclusion: Combining LA,aCL, aβ2GPI, and aPS/PT improves the diagnostic power and helps in stratifying the risk for each patient, according to their aPL profile
To cite this abstract in AMA style:
Sciascia S, Cecchi I, Radin M, Rubini E, Foddai S, Roccatello D, Bentow C, Seaman A, Ramirez C, Mahler M. Added Clinical Utility of Testing for Extra-Criteria Antibodies Specificities Beyond Sapporo and Sydney Criteria Recommendations [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/added-clinical-utility-of-testing-for-extra-criteria-antibodies-specificities-beyond-sapporo-and-sydney-criteria-recommendations/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/added-clinical-utility-of-testing-for-extra-criteria-antibodies-specificities-beyond-sapporo-and-sydney-criteria-recommendations/