Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) are life-threatening rare autoimmune diseases are both forms of anti-neutrophil cytoplasmic antibody-associated vasculitis (AAV). Standard of care treatment includes high-dose glucocorticoids (GC) in combination with cyclophosphamide (CYC) or rituximab (RTX). There is a high unmet medical need to reduce or eliminate high-dose GC to prevent patients from experiencing severe side effects. Complement component 5a (C5a) is known to prime and activate neutrophils, which is a major contributor to the pathogenesis of AAV. IFX-1, a monoclonal antibody, specifically binds to C5a inhibiting C5a-induced biological effects.
Methods: This clinical trial uses an adaptive, stepwise approach to evaluate if GC can be replaced by IFX-1 in AAV.
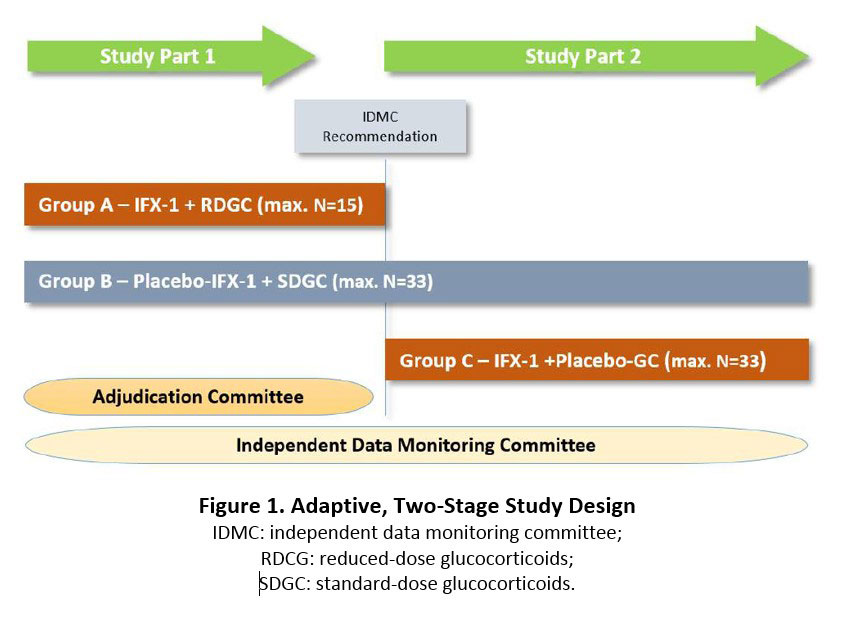
Results: This is a randomized, double-dummy, double-blind, multicenter trial evaluating the efficacy and safety of IFX-1 in subjects with moderate to severe GPA or MPA (ClinicalTrials.gov NCT03895801). The trial consists of two parts (Figure 1). All subjects in both parts are treated with RTX or CYC. In Study Part 1, subjects are randomized to receive either IFX-1 plus a reduced-dose of GC (Group A) or placebo-IFX-1 plus high-dose GC (Group B). Following global clinical evaluation, the trial proceeds to Part 2 in which either additional subjects are randomized to Group B (as above) or subjects are randomized to Group C: IFX-1 and placebo-GC. Patients in Group B are used as the comparison group in both Parts. In total, about 80 subjects will be randomized at approximately 90 sites in Europe and Russia.
Major eligibility criteria are a diagnosis of new or relapsing GPA or MPA requiring treatment with CYC or RTX plus GCs, known positive test for ANCA, ≥ 1 major item or ≥ 3 minor items or ≥ 2 renal items on the Birmingham Vasculitis Assessment Score version 3 (BVASv3).
Intravenous IFX-1 or corresponding placebo is given on days 1, 4, 8, 15 and then every other week until Week 16. GC or corresponding placebo is given according to a standardized tapering scheme. The primary endpoint is the proportion of subjects achieving clinical response, defined as ≥ 50% reduction in BVASv3 at Week 16 compared to baseline and no worsening in any body system. Key secondary endpoints include the clinical response at other time points, proportion of subjects with a clinical remission (BVASv3 = 0) at Week 16, adverse events, and pharmacokinetic and pharmacodynamics parameters.
An Adjudication Committee (AC) evaluates the treatment response of each subject after each study visit under blinded conditions. An unblinded Independent Data Monitoring Committee is responsible for continuous review of subject safety data and of reports from the AC to make a recommendation to continue with Study Part 2. Study enrollment started in April 2019.
Conclusion: This ongoing trial is designed to address the substantial unmet need for new therapies that allow for reducing the burden of GCs in the treatment of AAV. This adaptive design provides an innovative approach to investigating new agents in AAV that allows for testing smaller sample sizes in this rare disease while maintaining a strong focus on patient safety.
To cite this abstract in AMA style:
Merkel P, Hellmich B, Jayne D, Rückinger S, Tamas Z, Thielert C, Zenker O. Adaptive Study Design of a Randomized, Multicenter, 2-Part Phase 2 Trial of Replacement of Glucocorticoids by IFX-1, a C5a Inhibitor, in Active Granulomatosis with Polyangiitis and Microscopic Polyangiitis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/adaptive-study-design-of-a-randomized-multicenter-2-part-phase-2-trial-of-replacement-of-glucocorticoids-by-ifx-1-a-c5a-inhibitor-in-active-granulomatosis-with-polyangiitis-and-microscopic-polyang/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/adaptive-study-design-of-a-randomized-multicenter-2-part-phase-2-trial-of-replacement-of-glucocorticoids-by-ifx-1-a-c5a-inhibitor-in-active-granulomatosis-with-polyangiitis-and-microscopic-polyang/