Session Information
Date: Monday, November 9, 2015
Title: Miscellaneous Rheumatic and Inflammatory Diseases Poster Session II
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: To assess adalimumab (ADA) efficacy and safety in corticosteroid-dependent patients with inactive non-infectious, intermediate, posterior, or panuveitis enrolled in the international, double-masked trial, VISUAL II.
Methods: Patients aged ≥18 years with inactive, non-infectious, intermediate, posterior, or panuveitis requiring 10-35 mg oral prednisone to maintain an inactive state (no active, inflammatory chorioretinal and/or retinal vascular lesions, anterior chamber (AC) cell grade ≤0.5+ and vitreous haze (VH) grade ≤0.5+) were randomized 1:1 to receive placebo (PBO) or ADA (80 mg week 0, followed by 40mg every other week from week 1 for ≤80 weeks). From week 2, all patients underwent a mandatory prednisone taper to 0 mg by week 19 or earlier depending on the baseline dose. Primary endpoint was time to treatment failure (TF) in ≥1 eye at or after week 2. TF was defined as ≥1 of the following criteria: new active, inflammatory lesions; worsening of BCVA by ≥15 letters; 2-step increase in AC cell grade; 2-step increase in VH grade relative to baseline (BL). Secondary endpoints included change in AC cell grade, VH grade and BCVA from BL to the final visit, time to optical coherence tomography (OCT) evidence of macular edema (after week 2), and percent change in central retinal thickness (CRT) from BL to final visit. Adverse events (AEs) were monitored throughout the study.
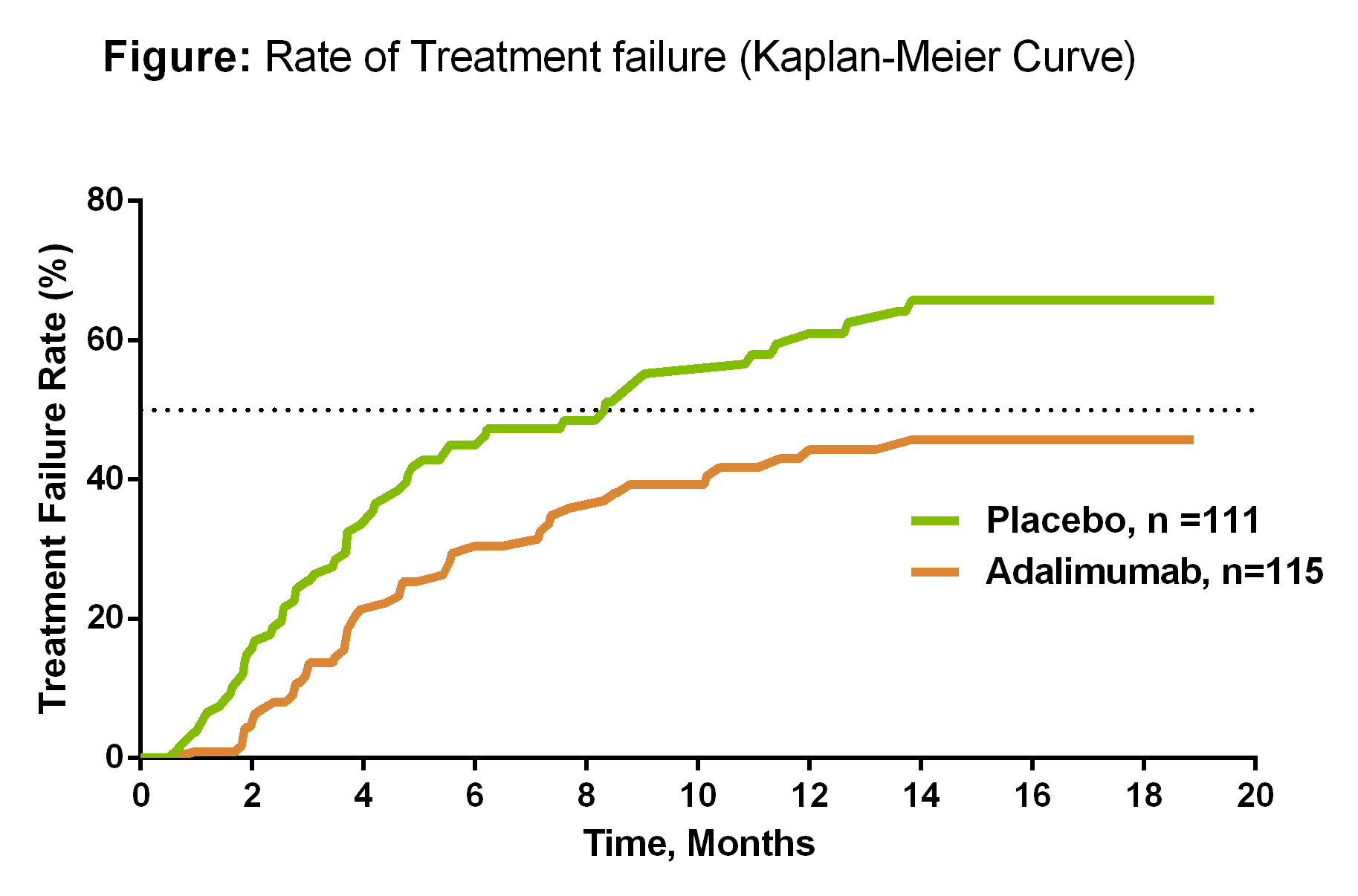
Results: A total of 229 patients were randomized (female, 61%; mean age, 42.5 years; mean duration of uveitis 61.2 months); 21% had intermediate, 32% had posterior, 46% had panuveitis, and 1% had intermediate and posterior uveitis. Patients who received ADA were less likely to have TF (hazard ratio=0.57; 95% CI, 0.39-0.84; P=0.004). Median time to TF was 8.3 months for PBO and not estimable for ADA, as more than half of the ADA-treated patients did not experience TF (Figure). Secondary endpoints were numerically in favor of ADA, although significant differences between ADA and PBO were not observed. No significant differences were observed between SAEs, serious infections and the overall rate of AEs between ADA and PBO.
Conclusion: ADA significantly lowered the risk for uveitic flare or vision loss in patients with steroid-dependent inactive, non-infectious uveitis. No new safety signals were identified with ADA treatment in patients with inactive uveitis; the safety profile of ADA in this population was comparable to other approved indications. Similar results were observed in patients with active, noninfectious, intermediate, posterior, or panuveitis despite the use of corticosteroids, enrolled in the VISUAL I trial.
To cite this abstract in AMA style:
Nguyen QD, Kurup SK, Merrill P, Sheppard J, Van Calster J, Dick AD, Jaffe G, Mackensen F, Rosenbaum JT, Schlaen A, Camez A, Tari S, Kron M, Song A, Brezin A. Adalimumab in Patients with Inactive, Non-Infectious Uveitis Requiring Systemic Treatment [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/adalimumab-in-patients-with-inactive-non-infectious-uveitis-requiring-systemic-treatment/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/adalimumab-in-patients-with-inactive-non-infectious-uveitis-requiring-systemic-treatment/