Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Research is redefining RA as a heterogeneous group of diseases distinguished by clinical, biochemical, and genomic markers. ACPA positivity is one such biomarker associated with a clinical profile of early rapidly progressing RA (ERPRA). This study aimed to understand patterns of biomarker testing including ACPA and their impact on treatment patterns in RA patients in the real-world practice setting.
Methods: A retrospective cohort study using chart review methodology was used to abstract data of adult RA patients treated in US community rheumatology clinics with bDMARD therapy ≤1 year of data collection launch (April 2019). Rheumatologists provided patient-level data from medical records including demographics, biomarker assessments, treatment sequencing, and clinical outcomes. Patient characteristics and outcomes were summarized descriptively.
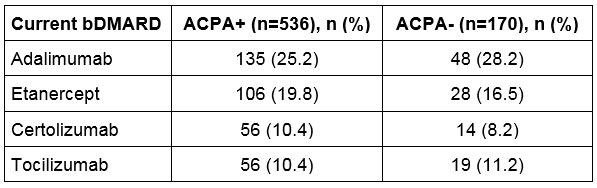
Results: There were 910 RA patients meeting eligibility criteria and abstracted between March 2018 and February 2019. Most patients were tested for ACPA status at RA diagnosis (77.6%). Of the ACPA tested patients, 75.9% were ACPA+ (anti-CCP2 concentration >19 AU/mL) and 24.1% were ACPA-. The proportion of patients retested for ACPA status was similar between the 2 cohorts (22.6% ACPA+ vs. 25.9% ACPA-, P=0.67). The majority of RA patients were female (73.7% ACPA+ vs. 75.9% ACPA-, P=0.57), Caucasian (74.3% ACPA+ vs. 80.6% ACPA-, P=0.09), and were covered by commercial health insurance (68.8% ACPA+ vs. 63.5% ACPA-, P=0.20). Although racial distribution was similar among ACPA+ and ACPA- cohorts, ACPA positivity was more prevalent in ACPA tested African Americans compared to ACPA tested Caucasians (87.1% vs. 74.4%, P< 0.01). ACPA+ patients were younger on average compared to ACPA- patients (48 years vs. 52 years, P< 0.01). There were no regional differences by ACPA status. The rate of testing of traditional biomarkers at the time of current bDMARD use: erythrocyte sedimentation rate 92.7%, C-reactive protein 79.1%, RF 98.9%; these rates did not differ by ACPA status (all P >0.05). Higher proportion of ACPA+ patients were RF+ compared to ACPA- patients (90.1% vs. 50.0%, P< 0.01). The treatment with bDMARD had similar distribution regardless of ACPA status as presented in the table.
Conclusion: These retrospective real-world data demonstrate that ACPA testing has attained good adoption among RA patients in US community rheumatology practices. ACPA positivity significantly varies from other biomarkers but does not appear to influence treatment choice. Additional research is needed to affirm published data that ACPA levels may differentiate disease that may be more effectively treated by non-TNF inhibitor bDMARDs.
To cite this abstract in AMA style:
Bapat B, Klink A, Kaufman J, Lobo F, Han X, Ferri L, Szymialis R, Poretta T, Feinberg B. ACPA Testing and Resultant Treatment Patterns in Patients with Rheumatoid Arthritis: Findings from US Community Rheumatology Practices [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/acpa-testing-and-resultant-treatment-patterns-in-patients-with-rheumatoid-arthritis-findings-from-us-community-rheumatology-practices/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/acpa-testing-and-resultant-treatment-patterns-in-patients-with-rheumatoid-arthritis-findings-from-us-community-rheumatology-practices/