Session Information
Session Type: Abstract Submissions (ACR)
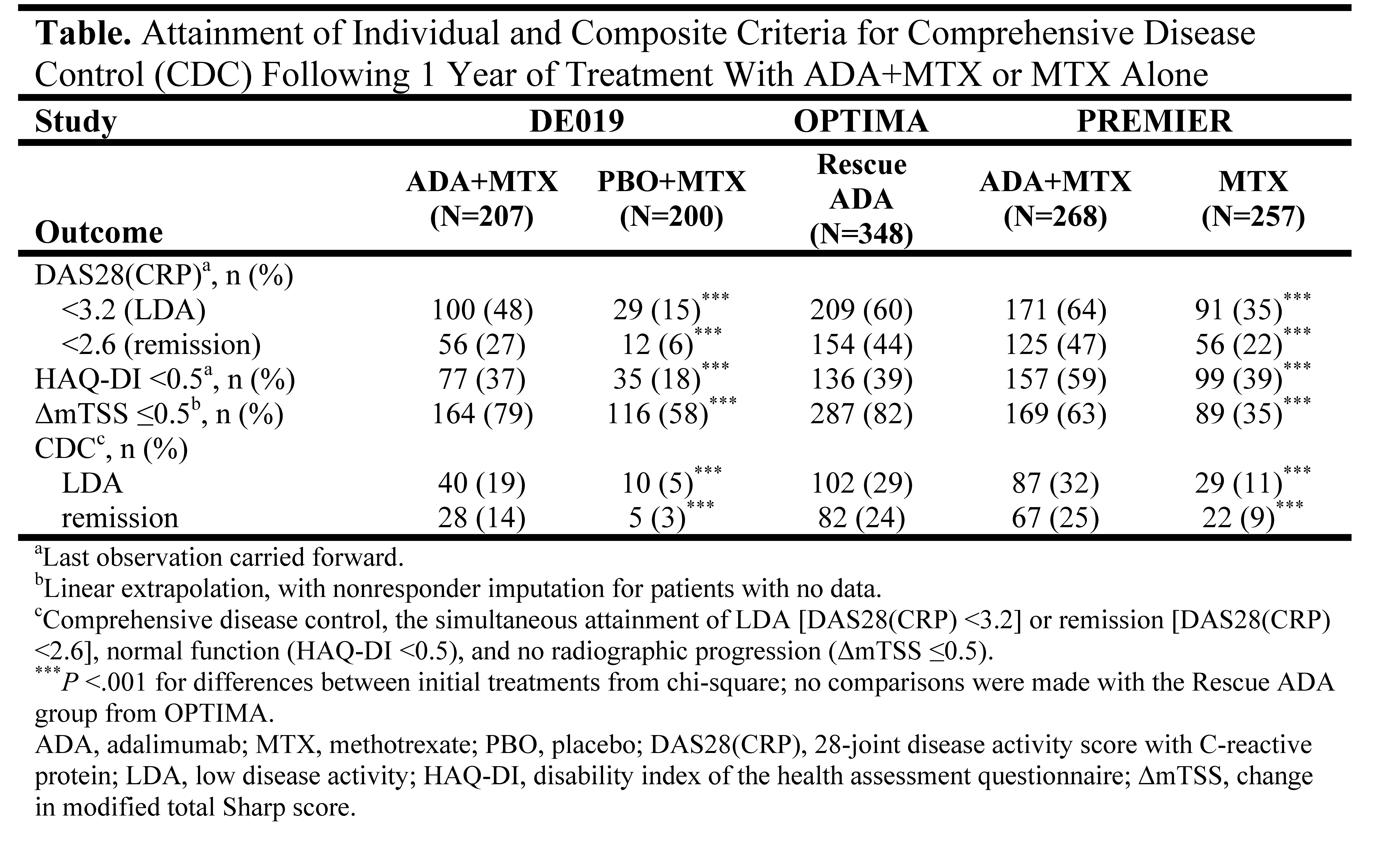
Background/Purpose: Effective treatment of rheumatoid arthritis (RA) patients (pts) aims to suppress inflammation, preserve physical function, and prevent structural damage, which together represent the hallmarks of comprehensive disease control (CDC).1 Advances in therapies and application of targeted approaches to disease management have made CDC a realistic treatment goal for many pts.2 The present analysis evaluated CDC attainment following 1 year of treatment with adalimumab (ADA) + methotrexate (MTX) vs MTX alone in 3 different randomized, controlled trials (RCTs).
Methods: Pt data originate from the DE019, OPTIMA, and PREMIER RCTs. DE019 enrolled pts with long-standing RA (mean =11 years) and an inadequate response (IR) to MTX; OPTIMA and PREMIER enrolled early RA (mean =0.4 and 0.7 years, respectively) and MTX-naïve pts. All studies included a comparison of ADA+MTX vs placebo (PBO)+MTX and were of at least 1 year duration. Changes to assigned treatment strategy were not allowed in DE019 or PREMIER but were made on the basis of achieving a stable low disease activity [LDA, DAS28(CRP) <3.2] target at weeks 22 and 26 in OPTIMA; PBO+MTX-IR could receive open-label (OL) ADA+MTX for an additional 52 weeks (Rescue ADA arm). CDC was defined for this analysis as the simultaneous achievement of LDA, normal function (HAQ-DI <0.5), and the absence of radiographic progression (ΔmTSS ≤0.5). This post hoc analysis assessed the proportion of pts achieving CDC and the individual criteria following 1 year of treatment with ADA+MTX combination therapy or MTX monotherapy in DE019, in the Rescue ADA arm of OPTIMA, or in PREMIER.
Results: Approximately one-fifth (19%) of ADA+MTX-treated pts in DE019 achieved CDC at 1 year vs 5% of PBO+MTX-treated pts (P <.001, Table). The addition of OL ADA+MTX to PBO+MTX-IR in the Rescue ADA arm of OPTIMA permitted 29% (n/N=102/348) of early RA pts to achieve CDC following 1 year of treatment, a proportion that was comparable with the proportion of MTX-naïve early RA pts treated with ADA+MTX in PREMIER achieving CDC at 1 year (32%, n/N=87/268). Significant differences between treatment groups in DE019 and PREMIER were still apparent when the disease activity component was replaced by DAS28(CRP) remission (<2.6).
Conclusion: CDC appears to be a realistic treatment goal in RA. Treatment with the combination of ADA+MTX led to significantly higher rates of CDC following 1 year than treatment with MTX alone, and earlier treatment appeared to increase the likelihood of CDC. A targeted treatment approach aiming at stable LDA at 6 months enabled MTX-IR who received OL ADA+MTX for an additional 1 year to achieve CDC in a proportion that was comparable with MTX-naïve early RA pts initiated with ADA+MTX, underscoring the utility of treat-to-target approaches in maximizing long-term outcomes.
References: 1. Smolen, et al. ARD 2010;69:631-7; 2. van der Heijde, et al. J Rheum 2010;37:2237-46.
Disclosure:
E. Keystone,
Abbott, Amgen, AstraZeneca, BMS, Centocor, Genzyme, Merck, Novartis, Pfizer, Roche, and UCB,
2,
Abbott, AstraZeneca, Biotest, BMS, Centocor, Genentech, Merck, Nycomed, Pfizer, Roche, and UCB,
5,
Abbott, Amgen, BMS, Janssen, Merck, Pfizer, Roche, and UCB,
8;
F. C. Breedveld,
Centocor, Schering-Plough, Amgen/Wyeth, and Abbott,
5;
D. van der Heijde,
Abbott, Amgen, AstraZeneca, BMS, Centocor, Chugai, Eli-Lilly, GSK, Merck, Novartis, Otsuka, Pfizer, Roche, Sanofi-Aventis, Schering-Plough, UCB, Wyeth,
5,
Imaging Rheumatology bv,
4;
R. F. van Vollenhoven,
Abbott, BMS, Glaxo SmithKline, Human Genome Sciences, Merck, Pfizer, Roche, and UCB Pharma,
5,
Abbott, BMS, Glaxo SmithKline, Human Genome Sciences, Merck, Pfizer, Roche, and UCB Pharma,
2;
S. Florentinus,
Abbott Laboratories,
1,
Abbott Laboratories,
3;
F. Faccin,
Abbott Laboratories,
1,
Abbott Laboratories,
3;
S. Liu,
Abbott Laboratories,
1,
Abbott Laboratories,
3;
H. Kupper,
Abbott Laboratories,
1,
Abbott Laboratories,
3;
A. Kavanaugh,
Abbott, Amgen, Astra-Zeneca, BMS, Celgene, Centocor-Janssen, Pfizer, Roche, and UCB,
2,
Abbott, Amgen, Astra-Zeneca, BMS, Celgene, Centocor-Janssen, Pfizer, Roche, and UCB,
5.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/achieving-comprehensive-disease-control-in-long-standing-or-early-rheumatoid-arthritis-patients-treated-with-adalimumab-plus-methotrexate-versus-methotrexate-alone/