Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Several disease activity measures and thresholds have been recommended as psoriatic arthritis (PsA) treatment targets, although consensus on the most appropriate assessment tool is lacking.1 Initial reports suggest low disease activity (LDA) and remission may be associated with minimal structural progression in PsA. Here we evaluate the relationship between PsA disease activity and structural progression over 216 weeks’ (wks’) treatment with certolizumab pegol (CZP), an Fc-free, PEGylated, anti-TNF that has shown long-term efficacy and safety in PsA.2
Methods: Patients (pts) enrolled in RAPID-PsA (NCT01087788) with active PsA (≥3 tender joints; ≥3 swollen joints; ESR≥28 mm/hour and/or CRP >upper limit of normal) who had failed treatment with ≥1 conventional synthetic DMARD were randomized 1:1:1 to CZP 200 mg every 2 wks (Q2W), CZP 400 mg every 4 wks (Q4W) (all CZP pts received CZP 400 mg at Wks 0/2/4) or placebo. CZP-randomized pts received the same dose to Wk 216; placebo-pts were re-randomized to CZP 200 mg Q2W or 400 mg Q4W at Wk 16 or 24.2
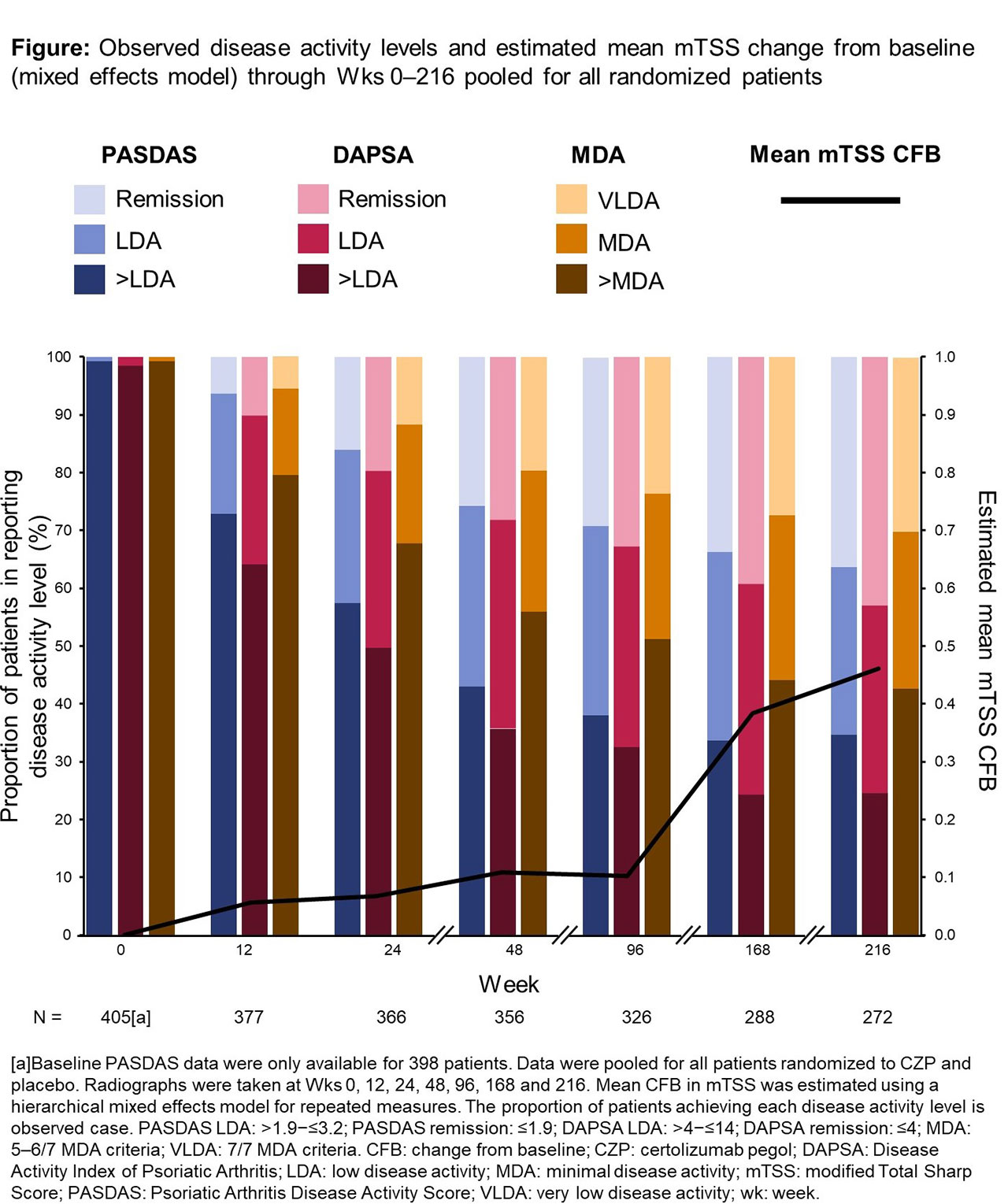
Pts were heterogenous for structural damage and disease duration at baseline. Disease activity was assessed using 3 disease activity measures: minimal disease activity (MDA) criteria (achievement of MDA=5–6/7 MDA criteria; achievement of very LDA [VLDA]=7/7 MDA criteria), Psoriatic Arthritis Disease Activity Score (PASDAS [LDA=score of >1.9–≤3.2; remission=≤1.9]), Disease Activity Index for Psoriatic Arthritis (DAPSA [LDA= >4–≤14; remission=≤4]). Radiographs taken through Wks 0–216 were read in 4 reading campaigns using van der Heijde modified Total Sharp Score (mTSS) for PsA. A subgroup of pts considered at highest risk of structural progression (baseline mTSS >median for all pts) was also assessed. Mean change from baseline (CFB) in mTSS, and associations with disease activity states, were estimated using a hierarchical linear mixed effects model (fixed effects: reading campaign, interactions of concurrent disease activity levels with time; random effects: pt, and reading campaign nested within pt) which allowed the trajectory of mean mTSS, and impact of disease activity levels on this, to be different between each timepoint at which radiographs were taken.
Results: Of 409 randomized pts, 407 were assessed for mTSS at least once. At Wk 0, mean (standard deviation) DAPSA was 44.5 (22.7), PASDAS was 6.0 (1.1). 3/409 (0.7%) pts reported MDA. The proportion of pts achieving remission/VLDA states increased to Wk 216, as did estimated mean mTSS, although overall progression was low (Wk 216 estimated mean mTSS CFB: 0.46; standard error: 0.16) (Figure). For all disease activity measures, remission/VLDA states were associated with mTSS estimated mean CFB ≤0 in both the overall group of pts and those considered at highest risk of structural progression at baseline (Table).
Conclusion: These data indicate that achievement of remission in PsA is important to prevent further structural damage, particularly when pts have pre-existing structural changes. This supports the rationale for strict disease activity targets.
References:
1. Coates L. Arthritis Rheumatol 2018;70:345–55; 2. van der Heijde D. RMD Open 2018;4:e000582.
To cite this abstract in AMA style:
Coates L, Merola J, Kavanaugh A, Mease P, Davies O, Irvin-Sellers O, Nurminen T, van der Heijde D. Achievement of Very Low Disease Activity and Remission Treatment Targets Is Associated with Reduced Radiographic Progression in Patients with Psoriatic Arthritis Treated with Certolizumab Pegol [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/achievement-of-very-low-disease-activity-and-remission-treatment-targets-is-associated-with-reduced-radiographic-progression-in-patients-with-psoriatic-arthritis-treated-with-certolizumab-pegol/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/achievement-of-very-low-disease-activity-and-remission-treatment-targets-is-associated-with-reduced-radiographic-progression-in-patients-with-psoriatic-arthritis-treated-with-certolizumab-pegol/