Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
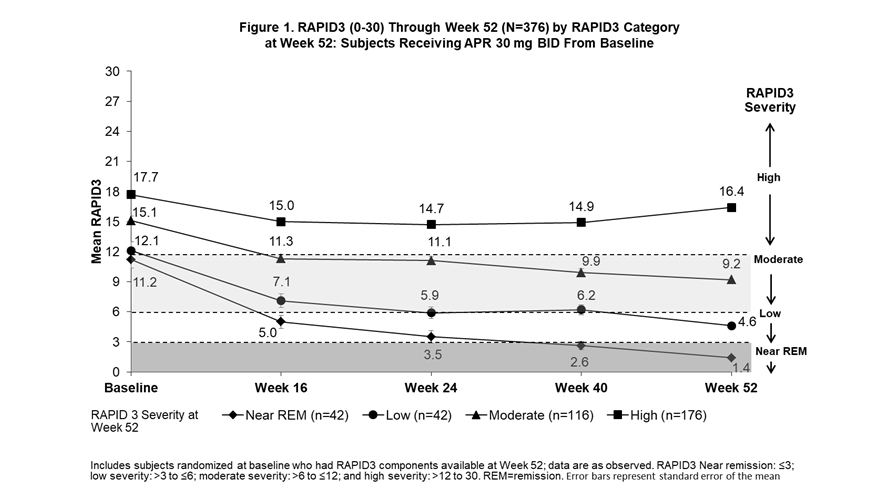
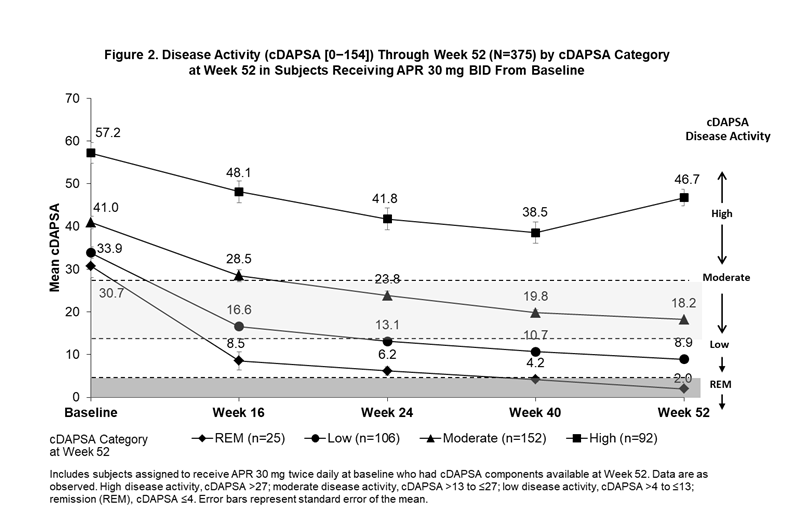
Background/Purpose: The Routine Assessment of Patient Index Data 3 (RAPID3) is an outcome measure of disease activity entirely derived from patient self-reported measures (Health Assessment Questionnaire-Disability Index [HAQ-DI] or multidimensional HAQ [MDHAQ], Pain visual analog scale [VAS], and Patient’s Assessment of Disease Activity [PtGA] VAS). The Clinical Disease Activity in Psoriatic Arthritis (cDAPSA; 0-154) includes objective and subjective physician assessments (i.e., a composite of swollen and tender joints counts [SJC and TJC]), along with Patient’s Assessment of Pain (PAP) and PtGA. In subjects receiving apremilast (APR), we examined trajectories for improvement in RAPID3 scores among subjects achieving RAPID3 near remission (REM) or low severity, cDAPSA among subjects achieving cDAPSA REM or low disease activity (LDA), and psoriatic arthritis (PsA) manifestations not measured by either outcome measure by Week 52.
Methods: Pooled analyses of the phase III PALACE 1, 2, and 3 studies were performed for subjects assigned to receive APR 30 mg BID at baseline (BL). Subjects with available scores on RAPID3 and cDAPSA components at Week 52 were included and grouped according to RAPID3 categories at Week 52 (near REM: ≤3; low severity: >3 to ≤6; moderate severity: >6 to ≤12; and high severity: >12 to 30) and cDAPSA categories at Week 52 (REM: ≤4; LDA: >4 to ≤13; moderate disease activity: >13 to ≤27; high disease activity: >27). Mean RAPID3 and cDAPSA scores were assessed from BL through Week 52. Other measures of PsA disease activity were reported longitudinally by RAPID3 and cDAPSA categories at Week 52.
Results: The RAPID3 and cDAPSA analysis included 376 and 375 APR subjects, respectively. Achievement of near REM or low severity (RAPID3) or REM or LDA (cDAPSA) by Week 52 with APR were associated with improvements over time in mean RAPID3 (Figure 1) and cDAPSA (Figure 2) trajectories. Subjects who achieved cDAPSA treatment targets were associated with no or mild articular and extra-articular manifestations at Week 52. Achieving RAPID3 treatment targets at Week 52 was associated with improvements in articular and extra-articular disease activity, although not all manifestations were controlled at Week 52 (Table). In both RAPID3 and cDAPSA analyses, similar improvements in SJC and TJC were observed for patients with REM or low severity (RAPID3) or REM or LDA (cDAPSA) at Week 52. In the RAPID3 analysis, mean TJC was higher than expected at Week 52, and achieving near REM RAPID3 scores was not associated with lower mean Psoriasis Area and Severity Index scores.
Conclusion: Subjects who achieved RAPID3 and cDAPSA targets showed early improvements in disease activity by Week 16 and sustained improvements to Week 52 with continued treatment. Achievement of treatment targets was also associated with improvements in other domains not captured directly by RAPID3 or cDAPSA. Given that some patients may exhibit different disease outcomes from the population, a more comprehensive assessment may help better evaluate treatment targets.
To cite this abstract in AMA style:
Bergman M, Yazici Y, Coates L, Smolen J, Husni M, Richter S, Teng L, Kavanaugh A. Achievement of RAPID3 and cDAPSA Treatment Targets Is Associated with Control of Articular and Extra-Articular Manifestations of Active Psoriatic Arthritis in Subjects Treated with Apremilast [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/achievement-of-rapid3-and-cdapsa-treatment-targets-is-associated-with-control-of-articular-and-extra-articular-manifestations-of-active-psoriatic-arthritis-in-subjects-treated-with-apremilast/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/achievement-of-rapid3-and-cdapsa-treatment-targets-is-associated-with-control-of-articular-and-extra-articular-manifestations-of-active-psoriatic-arthritis-in-subjects-treated-with-apremilast/