Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Guselkumab (GUS), a fully human IL-23p19-subunit inhibitor, has demonstrated multidomain efficacy (swollen/tender joints, psoriasis, enthesitis and dactylitis) in patients (pts) with active PsA in the phase 3, double-blind, placebo (PBO)-controlled DISCOVER (D)-1 and -2 studies.1,2 The clinical Disease Activity Index for PsA (cDAPSA) is a validated composite measure of joint disease activity in PsA, with established cut points for remission/low/moderate/high disease activity (REM/LDA/ModDA/HDA) states and is suitable for routine clinical practice. Pt-specific factors (sex, PsA duration, CRP, baseline disease activity/domain involvement) may influence PsA treatment efficacy.3 In a pooled analysis (D1+D2), cDAPSA LDA/REM was evaluated in pts with ModDA/HDA at baseline (BL) overall and in pt subgroups defined by BL demographic and disease characteristics.
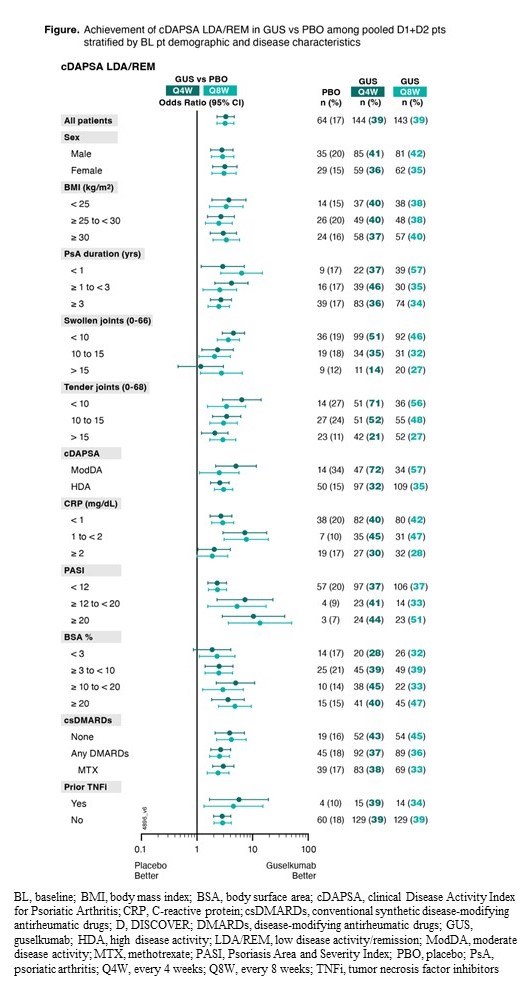
Methods: D1 (31% TNF inhibitor [i]-experienced) & D2 (biologic-naive) enrolled adults with active PsA despite conventional synthetic (cs) DMARDs. Pts were randomized 1:1:1 to GUS 100 mg every 4 weeks (Q4W); GUS 100 mg at Week (W)0, W4, then Q8W; or PBO→GUS 100 mg Q4W at W24. Pts with cDAPSA ModDA ( >13 to ≤27) or HDA ( >27) at BL were included in these post hoc analyses. Rates of cDAPSA LDA/REM (≤13) through W24 were determined using nonresponder imputation for missing data. Odds ratios (OR) and 95% CIs for achieving cDAPSA LDA/REM vs PBO were calculated using logistic regression and stratified by BL characteristics. Stratification factors were sex (male/female); BMI (< 25, ≥25 to < 30, ≥30); PsA duration (< 1, ≥1 to < 3, ≥3 years [y]); swollen/tender joint counts (SJC/TJC; < 10, 10 to 15, >15); cDAPSA (ModDA/HDA); CRP (< 1, 1 to < 2, ≥2 mg/dL); Psoriasis Area and Severity Index (PASI; < 12, ≥12 to < 20, ≥20); body surface area (BSA)% with psoriasis (< 3, ≥3 to < 10, ≥10 to < 20, ≥20); csDMARD use (none, any, MTX); prior TNFi (yes/no).
Results: Of 1120 D1+D2 pts, 947 (84.6%) with cDAPSA HDA and 166 (14.8%) with ModDA were included; 48-56% male/44-52% female, mean age: 46-47 y, mean PsA duration: 5.6-6.3, mean CRP: 1.6-1.9, mean cDAPSA: 44-45. W24 cDAPSA LDA/REM rates were significantly higher in GUS Q4W/Q8W pts vs PBO (38.7%/38.5%; vs 17.3%; both p≤0.0001); corresponding ORs (95% CI) vs PBO at W24: 3.3 (2.3-4.7) and 3.2 (2.2-4.6). GUS treatment effect vs PBO was consistent in subgroup of pts of adequate sample size defined by sex (OR: 2.8-3.2); BL BMI (OR: 2.5-3.7); PsA duration (OR: 2.5-6.4); SJC (OR: 1.2-4.5); TJC (OR: 2.1-6.4); cDAPSA (OR: 2.5-5.0); CRP (OR: 1.9-7.7); PASI (OR: 2.3-13.6); BSA% (OR: 1.9-5.0); concomitant csDMARD (OR: 2.6-4.2) and MTX (OR: 2.4-3.0); prior TNFi (OR: 2.8-5.7) (Figure).
Conclusion: Among PsA pts with highly active joint disease (cDAPSA ModDA/HDA) at BL, GUS-treated pts had significantly greater response rates vs PBO for achieving cDAPSA LDA/REM at W24. Both GUS dosing regimens showed consistent treatment effect in this pt population regardless of BL pt demographics, disease duration, joint and skin disease activity, or concomitant and prior PsA medication use.
1. Deodhar A. Lancet 2020;1115.
2. Mease P. Lancet 2020;395:1126.
3. Linde L. Rheumatology 2023; doi.org/10.1093/rheumatology/kead284.
To cite this abstract in AMA style:
Mease P, Gottlieb A, McInnes I, Shiff N, Todd A, Rampakakis E, Nantel F, Parrett J, Lavie F, Rahman P. Achievement of Low Disease Activity/Remission in Guselkumab-Treated Patients with Moderately-Highly Active Psoriatic Arthritis Regardless of Baseline Characteristics: Pooled Post-Hoc Analysis of Two Phase 3/Randomized Studies [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/achievement-of-low-disease-activity-remission-in-guselkumab-treated-patients-with-moderately-highly-active-psoriatic-arthritis-regardless-of-baseline-characteristics-pooled-post-hoc-analysis-of-two-p/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/achievement-of-low-disease-activity-remission-in-guselkumab-treated-patients-with-moderately-highly-active-psoriatic-arthritis-regardless-of-baseline-characteristics-pooled-post-hoc-analysis-of-two-p/