Session Information
Date: Saturday, November 7, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: The efficacy of ixekizumab (IXE), a selective interleukin-17A antagonist, was assessed in patients (pts) with axial SpA (axSpA) in three Phase 3, randomized, double-blind, placebo (PBO)-controlled trials, COAST-V, COAST-W and COAST-X. The BASDAI is frequently used by clinicians to measure disease activity and response to treatment in pts with axSpA, and when considering starting biologic DMARD therapy1,2. We present BASDAI and quality of life (QoL) outcomes at 16 weeks from the COAST trials.
Methods: COAST-V (NCT02696785) and COAST-W (NCT02696798) assessed pts with radiographic axSpA, and COAST-X (NCT02757352) assessed pts with non-radiographic axSpA. All pts fulfilled Assessment of Spondyloarthritis International Society (ASAS) classification criteria for axSpA. Pts were either biologic-naive (COAST-V, COAST-X) or TNFα inhibitor-experienced (COAST-W) and were randomized to IXE (80 or 160 mg at week 0 then 80 mg every 2 or 4 weeks [Q2W, Q4W]) or PBO or adalimumab (ADA, 40 mg Q2W; COAST-V only). Treatment response at week 16 was assessed by the proportion of patients achieving BASDAI< 4, 50% reduction from baseline in BASDAI (BASDAI50), and a clinically meaningful change from baseline in BASDAI of at least 2 units (ΔBASDAI≥2)1,2. Categorical variables were analyzed by logistic regression with non-responder imputation (NRI) for missing data. QoL was assessed by change from baseline in Short Form (SF)-36 Physical Component Summary (PCS) scores according to BASDAI< 4 response status at week 16; missing data were imputed using modified baseline observation carried forward (mBOCF).
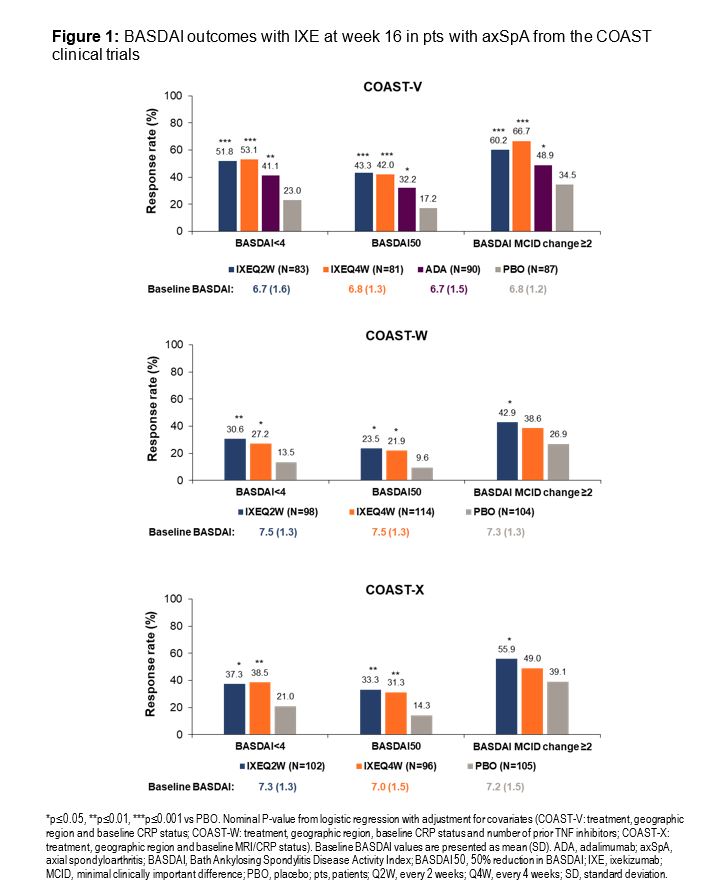
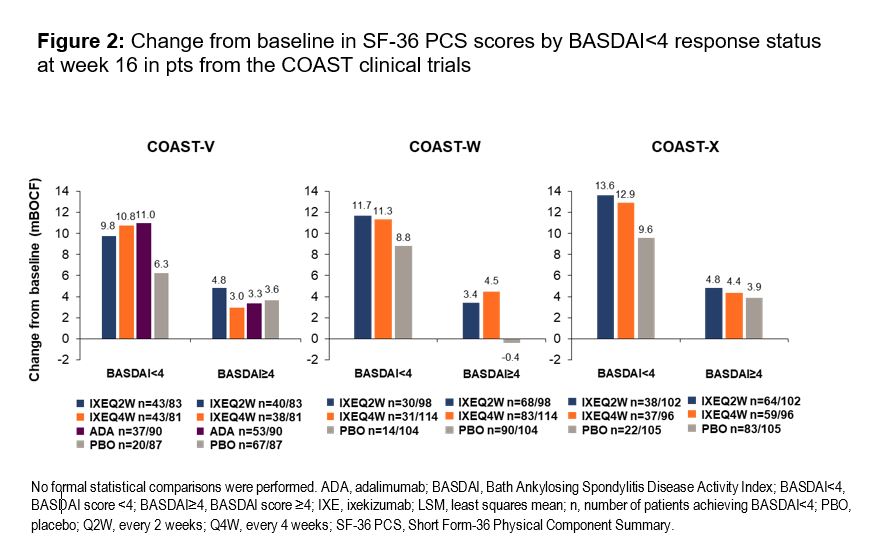
Results: In total, 341 pts from COAST-V, 316 from COAST-W, and 303 from COAST-X were included in this analysis. At week 16, a greater proportion of pts treated with IXE achieved BASDAI< 4, BASDAI50, and ΔBASDAI≥2 (Figure 1) compared to PBO across all three trials, and the difference was statistically significant for the majority of endpoints. Furthermore, pts achieving BASDAI< 4 showed greater improvements in SF-36 PCS scores (Figure 2).
Conclusion: In the COAST trials, IXE delivered clinically meaningful improvements in pts with axSpA after 16 weeks of treatment. Low disease activity (BASDAI< 4) was achieved with IXE regardless of axSpA type (radiographic or non-radiographic) or prior use of TNFα inhibitors. Achieving BASDAI< 4 was associated with greater improvements in physical QoL.
- Magrey MN, Kiltz U. Chapter 9 in Axial Spondyloarthritis. Elsevier, 2019:121-133.
- van der Heijde D, et al. Ann Rheum Dis 2017;76:978-991.
To cite this abstract in AMA style:
Poddubnyy D, Juanola X, Prati C, Russ H, Schymura Y, Liu-Leage S, Haschemi Nassab M, Dudler J. Achievement of Low Disease Activity According to BASDAI with Ixekizumab in Patients with Axial Spondyloarthritis: 16-Week Results from the COAST Trials [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/achievement-of-low-disease-activity-according-to-basdai-with-ixekizumab-in-patients-with-axial-spondyloarthritis-16-week-results-from-the-coast-trials/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/achievement-of-low-disease-activity-according-to-basdai-with-ixekizumab-in-patients-with-axial-spondyloarthritis-16-week-results-from-the-coast-trials/