Session Information
Date: Monday, October 22, 2018
Title: Spondyloarthritis Including Psoriatic Arthritis – Clinical Poster II: Clinical/Epidemiology Studies
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Therapeutic targets for psoriatic arthritis (PsA) include the achievement of remission (REM) or low disease activity (LDA), measured by the Clinical Disease Activity Index for Psoriatic Arthritis (cDAPSA), a composite of swollen and tender joints counts (SJC and TJC), Patient’s Assessment of Pain (PAP), and Patient’s Global Assessment of Disease Activity (PtGA). We examined the trajectories for improvement in cDAPSA and PsA manifestations not measured by cDAPSA among subjects achieving cDAPSA LDA or REM at Week 52.
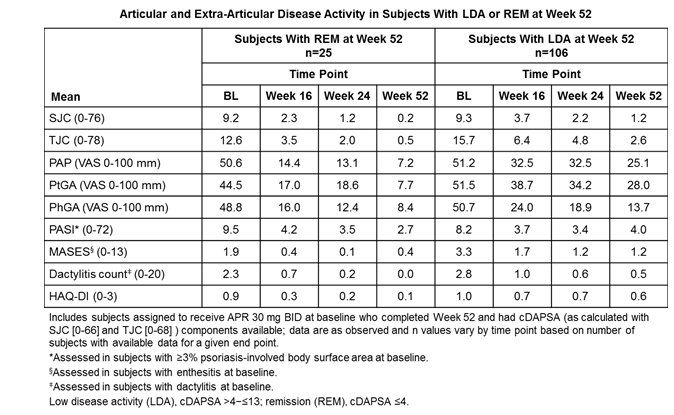
Methods: Pooled analyses of 3 phase III studies (PALACE 1-3) were performed for subjects assigned to receive apremilast 30 mg BID (APR) at baseline (BL). Subjects with cDAPSA components available to calculate responses at Week 52 were included and grouped according to the cDAPSA categories reached at Week 52 (REM: ≤4; low: >4 to ≤13; moderate: >13 to ≤27; high: >27 disease activity). We then traced their mean cDAPSA trajectory from BL to Week 52. Mean disease activity in core PsA domains were also reported longitudinally by cDAPSA category reported at Week 52, including SJC (0-76), TJC (0-78), PAP (visual analog scale [VAS] 0-100 mm), PtGA (VAS 0-100 mm), Physician’s Global Assessment of Disease Activity (PhGA; VAS 0-100 mm), Psoriasis Area and Severity Index (PASI; 0-72), enthesitis (Maastricht Ankylosing Spondylitis Enthesitis Score [MASES]; 0-13), dactylitis count (0-20), and HAQ-DI (0-3).
Results: A total of 375 APR subjects were included in the analyses. Achievement of LDA or REM at Week 52 was associated with lower mean cDAPSA at BL, and these subjects had continuous improvements in disease activity from BL to Week 52 (Figure). Among subjects who achieved LDA at Week 52, most were classified as having moderate (mean cDAPSA: 16.6) or low (mean cDAPSA: 8.5) disease activity at Week 16. At Week 24, these subjects had mean cDAPSA scores of 13.1 (moderate disease activity) and 6.2 (LDA). Furthermore, subjects who achieved REM at Week 52 had already shown early improvement to either no or mild articular and extra-articular disease activity by Week 16. Patients in REM and LDA showed parallel improvements in extra-articular disease activity at Week 52 with APR (Table).
Conclusion: In the subgroup who achieved cDAPSA REM or LDA, early improvement was seen in disease activity by Week 16 and sustained to Week 52 with continued treatment. Patients achieving control of peripheral arthritis (classified as REM or LDA by Week 52) with APR also exhibited REM or LDA in other manifestations of PsA, including enthesitis, dactylitis, function, and skin psoriasis.
To cite this abstract in AMA style:
Coates LC, Mease PJ, Behrens F, Orbai AM, Ogdie A, Brunori M, Teng L, Guerette B, Smolen JS. Achievement of Cdapsa Low Disease Activity or Remission Is Associated with Control of Articular and Extra-Articular Manifestations of Active Psa in Subjects Treated with Apremilast [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/achievement-of-cdapsa-low-disease-activity-or-remission-is-associated-with-control-of-articular-and-extra-articular-manifestations-of-active-psa-in-subjects-treated-with-apremilast/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/achievement-of-cdapsa-low-disease-activity-or-remission-is-associated-with-control-of-articular-and-extra-articular-manifestations-of-active-psa-in-subjects-treated-with-apremilast/