Session Information
Date: Sunday, November 13, 2022
Title: RA – Treatment Poster II
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Little is known about the duration of humoral antibody levels after two SARS-CoV-2 mRNA vaccinations in patients with immunosuppression. During this ongoing global epidemic, it is of essential interest to gather information about the time of protection after initial immunization in the vulnerable patients receiving either conventional synthetic disease modifying antirheumatic drugs (csDMARD) or biological/ targeted drugs (b/tsDMARDs).
In this study we compared the antibody level development after vaccination and after six months in patients with inflammatory arthritis, inflammatory bowel disease (IBD) and healthy controls. Furthermore, we assessed factors affecting the quality and quantity of the humoral response.
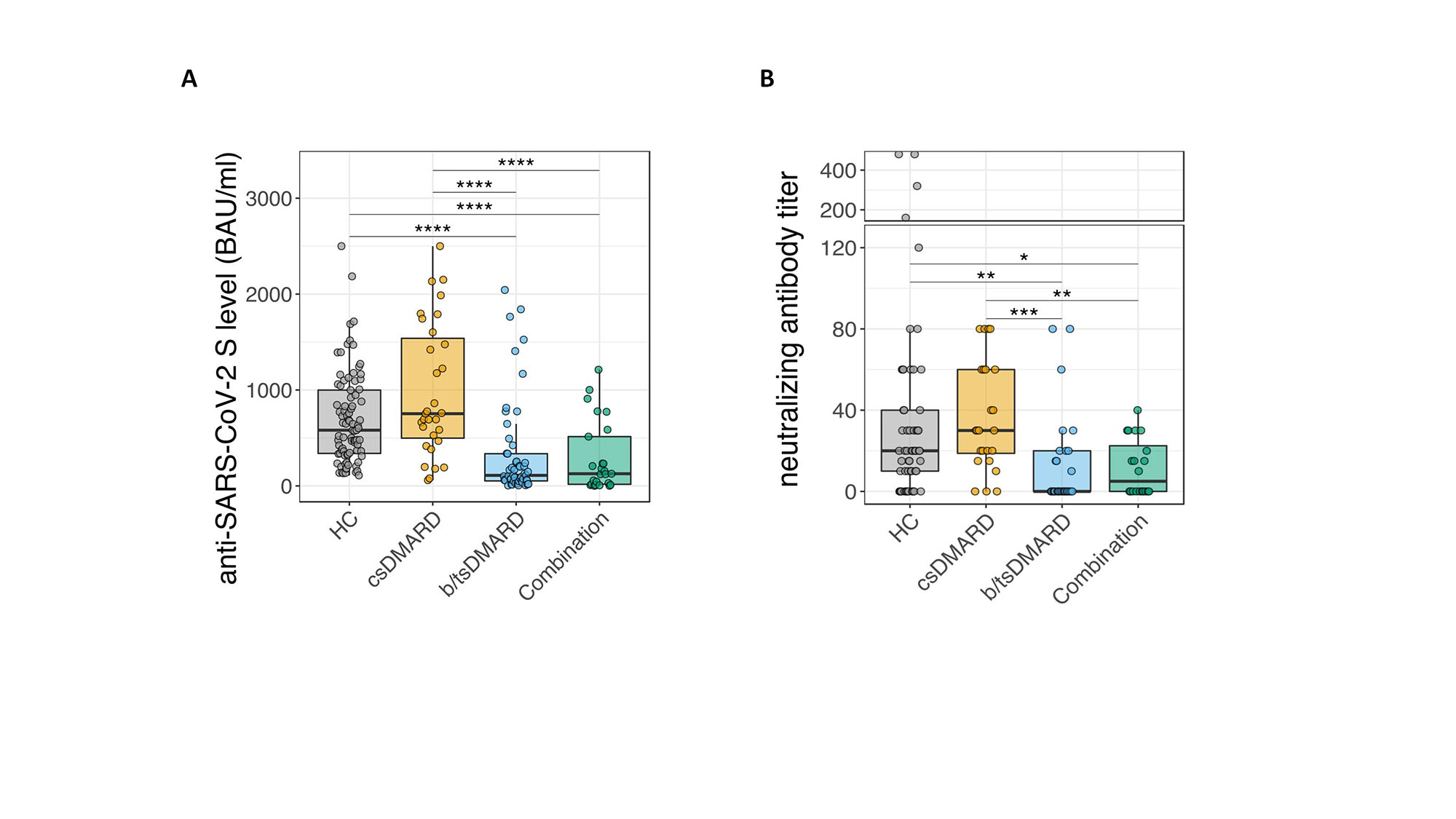
Methods: We enrolled 85 healthy controls (HC), 75 patients with rheumatoid arthritis and spondyloarthritis and 41 patients suffering from IBD. Patients treated with B-cell depleting therapies were excluded from this study. Binding antibody units were measured after vaccination and 6 or more months. Neutralizing antibodies were measured after 6 months. Multivariate regression analyses analyzing factors associated with low titers after 6 months was performed.
Results: We found that patients with inflammatory arthritis or IBD showed reduced anti-SARS-CoV-2 S titers compared to HC. When we stratified for therapies, we found that patients receiving conventional synthetic disease modifying antirheumatic dugs (csDMARDs) had comparable anti-SARS-CoV-2 S titers to HC. In contrast, patients receiving biological or targeted synthetic (b/tsDMARDs) showed reduced anti-SARS-CoV-2 Igs as well as neutralizing antibody titers compared with healthy controls (HC) or patients receiving conventional synthetic (cs)DMARDs. We further show that anti-SARS-CoV-2 titers declined more rapidly in patients receiving b/tsDMARDs compared to HC, leading to a 50 percent reduction in vaccination-associated protection time in patients receiving b/tsDMARDs when compared to those receiving csDMARDs or even HC. In multivariate regression analyses, we find that in addition to the type of treatment, also age as well as corticosteroid use were associated with reduced anti-SARS-CoV-2 S titer.
Conclusion: Patients under ongoing b/tsDMARDs therapy exposed an accelerated waning of anti-SARS-CoV-2 S titers and therefore decreased immunity and protection against severe Covid-19 infections over time. These results may lead to more personalized approaches for further vaccination strategies in this group of immunosuppressed patients.
To cite this abstract in AMA style:
Simader E, Tobudic S, Deimel T, mandl P, Haslacher H, Perkmann T, Schneider L, Nothnagl T, Radner H, Winkler F, Burgmann H, Stiasny K, Novacek G, Reinisch W, Aletaha D, Winkler S, BLUEML S. Accelerated Waning of Protective Immunity After SARS-CoV-2 mRNA Vaccination in Patients Treated with Biological and Targeted Synthetic Disease Modifying Antirheumatic Drugs [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/accelerated-waning-of-protective-immunity-after-sars-cov-2-mrna-vaccination-in-patients-treated-with-biological-and-targeted-synthetic-disease-modifying-antirheumatic-drugs/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/accelerated-waning-of-protective-immunity-after-sars-cov-2-mrna-vaccination-in-patients-treated-with-biological-and-targeted-synthetic-disease-modifying-antirheumatic-drugs/