Session Information
Date: Monday, November 6, 2017
Title: Rheumatoid Arthritis – Clinical Aspects II: Treatment Patterns
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: In the absence of biomarkers predicting response to a specific therapy, the choice of second biologic is based mostly on habit and availability of an alternative agent. Traditionally, a second anti-TNF was the preferred option, but recent registry data point to better responses and retention if a drug with a different mode of action is prescribed. The aim of this study was to assess the long-term retention of abatacept and TNF inhibitor (TNFi) following first biologic (b)DMARD inadequate response in RHUMADATA® registry patients with RA.
Methods: Data from RHUMADATA® patients with RA prescribed either abatacept or TNFi as the second bDMARD after January 1, 2006 were analyzed. Patients were followed until treatment discontinuation or January 9, 2017 cut-off. Patient characteristics were compared using descriptive statistics, bDMARD discontinuation rates using Kaplan–Meier methods, and proportional hazard models were used to identify predictors of treatment discontinuation.
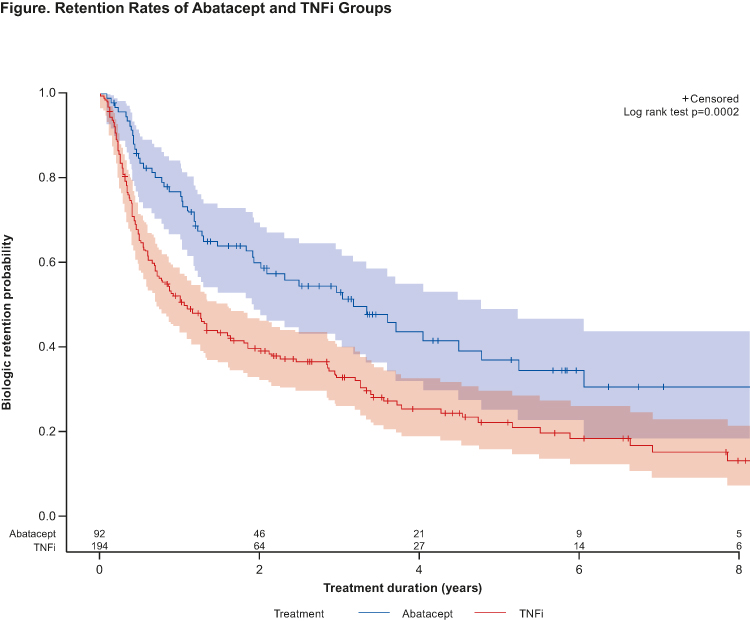
Results: Data for 92 and 194 patients prescribed abatacept or a TNFi, respectively, as second-line treatment were extracted. No clinically significant differences in baseline characteristics were noted between treatment groups. Most patients were women (76.2%), average age (SD) was 45.1 (13.3) years at diagnosis and disease duration 10.8 (9.0) years. Most patients were stopping an anti-TNF agent: 83% of those who were switched to abatacept and 97% of those who were prescribed a second anti-TNF. Overall, 77.6% of patients stopped their first bDMARD after >6 months of treatment (secondary failure). Significant differences in retention between abatacept and TNFi groups (logrank p=0.0002) were observed (Table, Figure). Results remained unchanged for patients treated with TNFi only in first line, and primary/secondary failure of the first bDMARD did not affect sustainability of the second agent. Lack of efficacy (57.7%) and AEs (16.5%) were the most commonly cited reasons for treatment discontinuation.
Conclusion: Abatacept has better sustainability over a second line TNFi in RA patients having failed one prior bDMARD.
Original abstract © EULAR/BMJ. First presented at EULAR 2017 and published in Ann Rheum Dis 2017;76 (Suppl 2):AB0397. Any reprints, promotional options, education material etc have to be done through the original source (ARD/BMJ).
| |
||||||
| |
|
|||||
| |
|
|
||||
| |
|
|
|
|
||
| |
|
|
|
|
||
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
||||||
| |
|
|
||||
| |
|
|
||||
| |
|
|
||||
| |
|
|
||||
| |
|
|
||||
| |
||||||
| |
|
|
||||
| |
|
|
||||
| |
|
|
||||
| |
|
|
||||
| |
||||||
To cite this abstract in AMA style:
Choquette D, Bessette L, Alemao E, Haraoui B, Massicotte F, Mtibaa M, Muratti E, Pelletier JP, Postema R, Raynauld JP, Rémillard MA, Sauvageau D, Turcotte A, Villeneuve É, Coupal L. Abatacept Shows Better Sustainability Than TNF Inhibitors When Used Following Initial Biologic DMARD Failure in the Treatment of RA: 8 Years of Real-World Observations from the Rhumadata® Clinical Database and Registry [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/abatacept-shows-better-sustainability-than-tnf-inhibitors-when-used-following-initial-biologic-dmard-failure-in-the-treatment-of-ra-8-years-of-real-world-observations-from-the-rhumadata-clinica/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/abatacept-shows-better-sustainability-than-tnf-inhibitors-when-used-following-initial-biologic-dmard-failure-in-the-treatment-of-ra-8-years-of-real-world-observations-from-the-rhumadata-clinica/