Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
Traditional DMARDs have limited effects in primary Sjögren’s (pSS) patients. B cell depletion therapy with rituximab showed efficacy, but had some side-effects. T and B cells are known to play a critical role in the pathophysiology of pSS. Abatacept, a selective co-stimulation modulator of T lymphocytes, could be an alternative treatment option for pSS. In this study the efficacy and safety of abatacept in early pSS patients was assessed.
Methods:
All patients (12 female, 3 male) included in the open-label Active Sjögren Abatacept Pilot (ASAP) study met the revised American-European Consensus Group criteria. Disease duration was <5 years. Traditional DMARDs, hydroxychloroquine or corticosteroids were discontinued ≤ 1 month before baseline. Patients were biological DMARD naïve, and were treated with 8 abatacept infusions (≈10 mg/kg of body weight) administered intravenously on days 1, 15, and 29 and then every 4 weeks thereafter (total treatment period 24 weeks). Follow-up was conducted at 4, 12, 24 (on treatment), 36 and 48 weeks (off treatment). Disease activity was assessed with EULAR Sjögren's Syndrome Disease Activity Index (ESSDAI) and Patient Reported Index (ESSPRI). Several other functional, laboratory and subjective variables were analysed. Parotid gland biopsies were taken at baseline and week 24. Generalized estimating equations were used to analyse parameters over time.
Results:
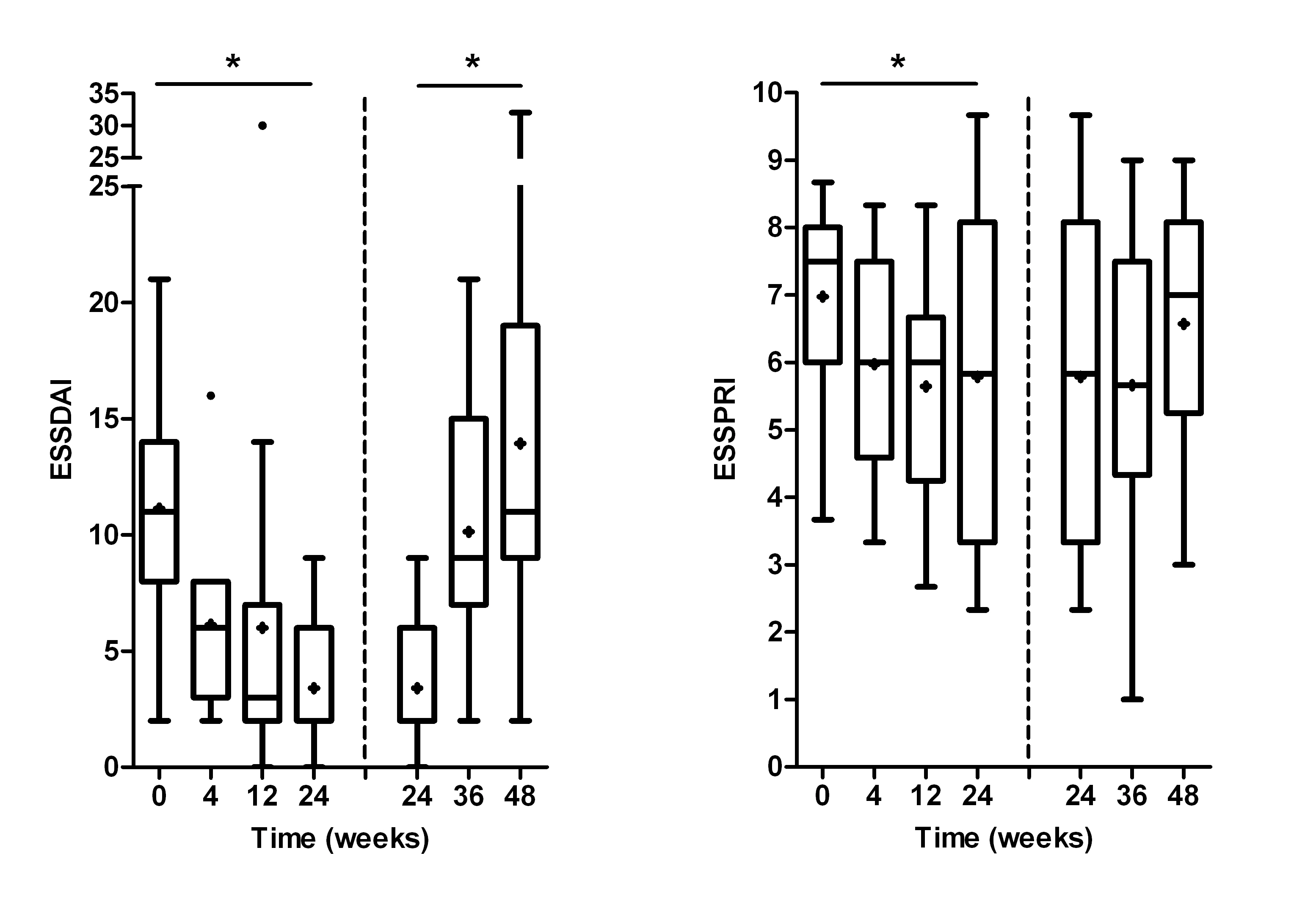
Median age and disease duration were 43 years and 11 months, respectively. Median ESSDAI decreased significantly from 11 (range 2-21) before abatacept to 2 (range 0-9) 24 weeks after abatacept (p<0.000) (Figure 1). ESSDAI increased significantly post-treatment (p<0.000). Median ESSPRI decreased significantly from 7.7 (range 3.7-8.7) to 5.8 (range 2.3-9.7) during treatment (p=0.015), followed by a trend towards increased ESSPRI post-treatment. Similar patterns were found for rheumatoid factor and IgG levels. Salivary and lacrimal gland function remained stable during treatment. Absolute numbers of T and B cells changed slightly (<12%). Fatigue and health-related quality of life improved significantly during treatment. No serious side effects or infections were seen. Histological findings are currently analysed and will be presented at the ACR meeting.
Conclusion:
This open label pilot study indicates that abatacept is well tolerated, safe and results in reduced disease activity, laboratory and subjective parameters in pSS patients. Most patients experienced a clinically relevant improvement in their well-being. These very promising results warrant confirmation in a randomized, double-blind, placebo-controlled clinical trial.
Figure 1. Change in disease activity over time, on treatment (0-24 weeks) and off treatment (24-48 weeks). Box-and-whisker plots (Tukey); boxes=medians with interquartile ranges; +=means; whiskers=1.5 times the interquartile distances; •=outliers. *=p<0.05 over time.
Disclosure:
P. Meiners,
BMS,
2;
A. Vissink,
BMS,
2;
F. Spijkervet,
BMS,
2;
E. Haacke,
BMS,
2;
W. Abdulahad,
BMS,
2;
E. Brouwer,
BMS,
2;
M. Huitema,
BMS,
2;
N. Sillevis Smitt-Kamminga,
BMS,
2;
F. Kroese,
BMS,
2;
S. Arends,
BMS,
2;
H. Bootsma,
BMS,
2.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/abatacept-reduces-disease-activity-in-early-primary-sjogrens-syndromeone-year-results-from-a-phase-ii-open-label-study/