Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Abatacept (ABA) is a fusion protein of the extracellular domain of CTLA4 and human IgG1-Fc, constructed to inhibit B/T cell co-stimulation. Previous studies of ABA in lupus failed to show benefit, but improvement in arthritis was suggested. Those studies included significant background medications and steroid rescue. The current 6-month randomized, double-blind, placebo (PBO)-controlled study of ABA in patients with SLE arthritis withdrew background medications to facilitate assessments.
Methods: Patients were entered with moderate to severe arthritis (BILAG A or B with ≥3 swollen and ≥3 tender joints). All SLE treatment except low dose prednisone was withdrawn and patients were randomized 1:1 to weekly sc ABA or PBO. DepoMedrol injections (≤320mg total) were allowed, if needed, until Month 2. At Month 3, additional steroids, immune suppressants or open-label ABA were allowed, but designated non-response. The primary endpoint was the BILAG-based Combined Lupus Assessment (BICLA) response at Month 6. The SELENA-SLEDAI PGA (SSPGA) and the LFA-Rapid Evaluation of Activity in Lupus (LFA-REAL) were used as exploratory endpoints. The SSPGA is a single visual analogue scale (VAS) assessing overall lupus activity, and the LFA-REAL derives a total VAS score by summing individual symptoms (1).
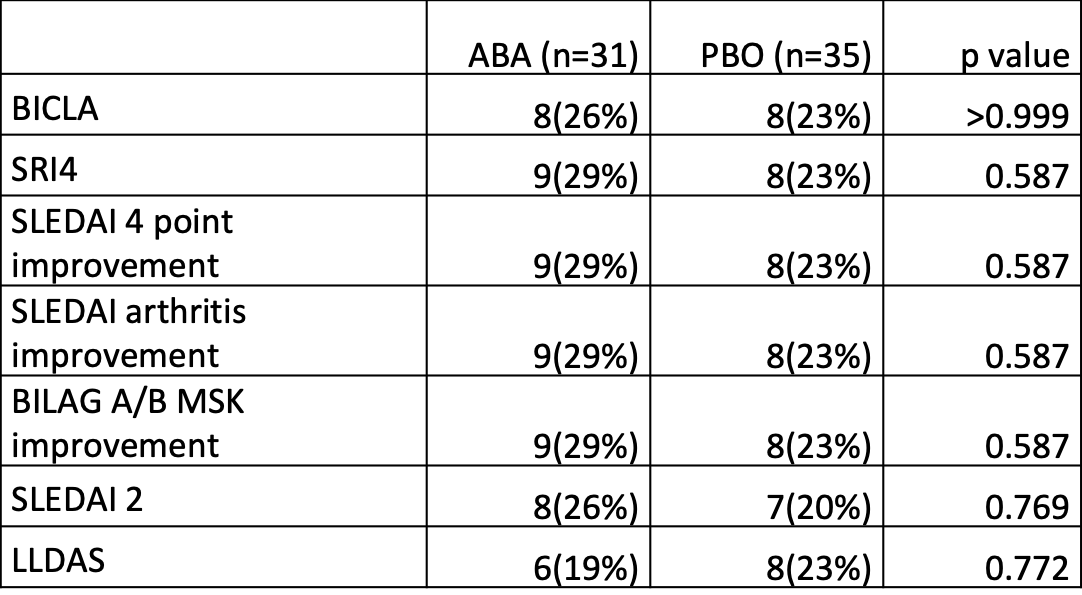
Results: 66 randomized patients received at least one dose of the study drug. Placebo response rates were lower than most SLE trials, but no primary or secondary endpoints were met (Table). The safety profile of ABA was consistent with its known effects. Additionally, a flare analysis was performed.
By Month 6, 13 (42%) patients on ABA vs. 15 (45%) PBO had a moderate/severe flare and 22 (71%) patients on ABA vs. 22 (67%) PBO experienced flare or treatment failure (dropout or added medications) (Log-Rank test, p=0.941 and p=0.539, respectively). Using the modified SELENA-SLEDAI Flare Index (mSSFI) (2) or BILAG flare index, global flare severity did not differ between groups, even after adjusting for rescue steroids, SLEDAI at screening and SLEDAI at visit prior to flare. Similarly, BILAG musculoskeletal (MSK) flares did not differ between groups.
Using the SSPGA and LFA-REAL, flare severity was greater in the PBO group vs. the ABA group. Adjusting for LFA-REAL MSK score at visit prior to flare and flare severity by BILAG MSK, increase in LFA-REAL MSK at flare visits was 8.7±1.5 for PBO and 3.0±1.5 for ABA (p=0.07). Adjusting for SSPGA at visit prior to flare and flare severity by mSSFI, increase in SSPGA at flare visits was 10.4±1.6 for PBO and 4.8±1.7 for the ABA group (p=0.017). The score difference for LFA-REAL was 15.5±2.7 for PBO vs. 5.1±2.8 for ABA-treated patients (p=0.008).
Conclusion: This protocol lowered PBO response rates to ≤23% by mandating withdrawal of background treatments. ABA did not demonstrate improvement in controlling disease activity. SSPGA and LFA-REAL scores suggested a decrease in flare severity in the ABA-treated patients.
References:
1. Askanase A, Li X, Pong A, Shum K, Kamp S, Carthen F, Aberle T, Hanrahan L, Daly P, Giles J, Merrill JT. Lupus Sci Med. 2015 Mar 4;2(1):e000075.
2. Thanou A, Chakravarty E, James JA, Merrill JT. Rheumatology (Oxford) 2014;53(12):2175-81.
To cite this abstract in AMA style:
Thanou A, Arriens C, Aberle T, Miller H, Mitchell L, Kamp S, Askanase A, Stavrakis S, James J, Merrill J. Abatacept Failed to Demonstrate Efficacy in an SLE Trial with Low Placebo Response Rates, Although Global Assessments Indicated Less Flare Severity [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/abatacept-failed-to-demonstrate-efficacy-in-an-sle-trial-with-low-placebo-response-rates-although-global-assessments-indicated-less-flare-severity/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/abatacept-failed-to-demonstrate-efficacy-in-an-sle-trial-with-low-placebo-response-rates-although-global-assessments-indicated-less-flare-severity/