Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Previous randomized controlled studies reported conflicting results regarding the effectiveness of colchicine on oral ulcers in Behçet syndrome (BS). A randomized drug discontinuation design is a good study scheme for evaluating efficacy. We aimed to assess mucocutaneous disease activity after 12 weeks in BS patients who discontinued colchicine.
Methods: This is a single centre, randomized, controlled, single-blind trial (NCT06146192). BS patients with mucocutaneous and joint disease but no major organ involvement for at least 2 years who fulfilled ISG criteria and who were using colchicine at least 1 mg/day were included. An independent observer who was blinded to group allocations performed all assessments. At the end of a 12-week observational period during which all patients continued to use colchicine, patients were randomized to continue or withdraw colchicine for 12 weeks. Randomization was stratified (1:1) according to disease activity (having active disease vs having minimal disease activity), disease duration, and sex. Primary endpoint was the mean number of oral ulcers at week 12 after randomization. Secondary endpoints included the number of genital ulcers, erythema nodosum-like lesions, papulopustular lesions, pain of oral ulcers, genital ulcers, and erythema nodosum-like lesions, overall disease activity assessed using Behçet’s disease current activity form (BDCAF) and Behçet’s syndrome activity scale (BSAS), and quality of life assessed using Behçet disease quality of life index (BDQoL), and SF-36.
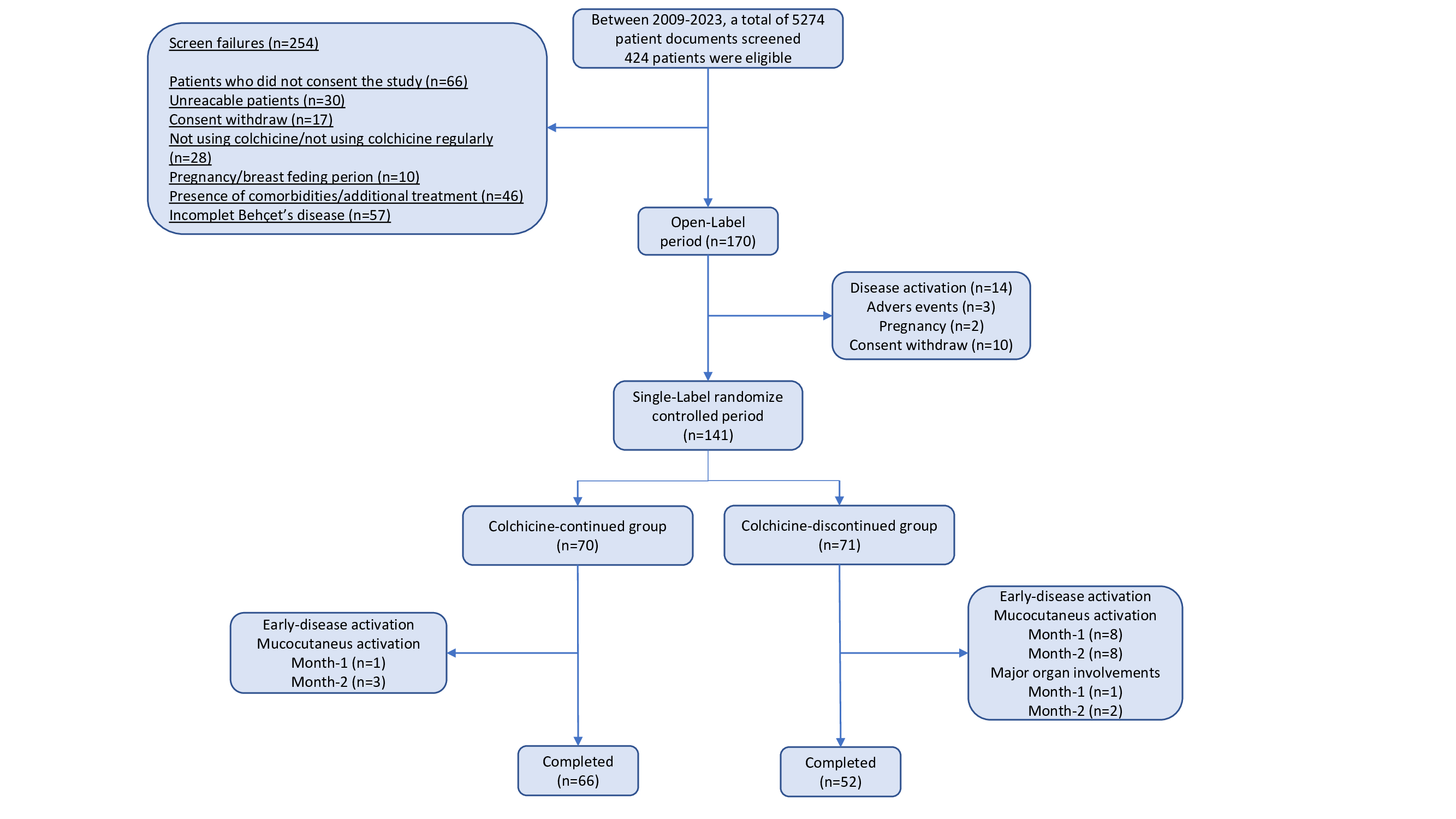
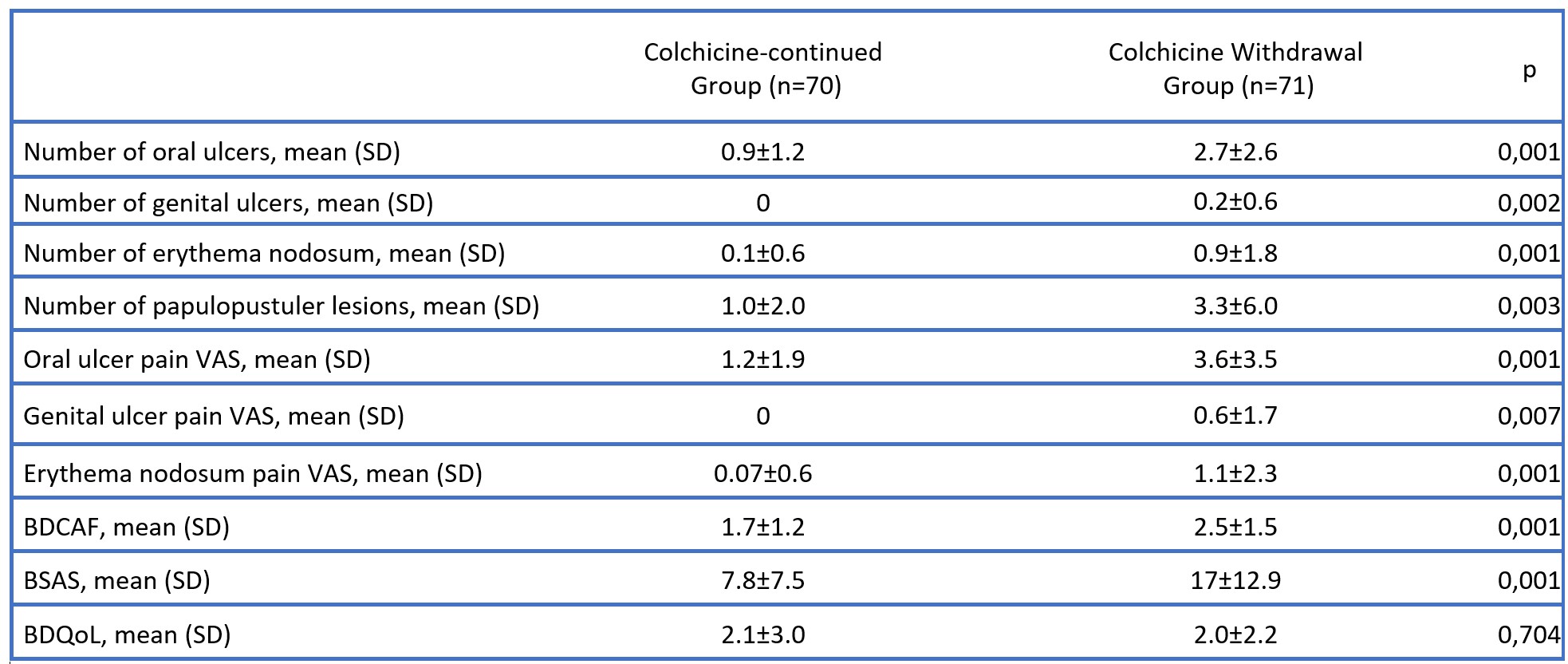
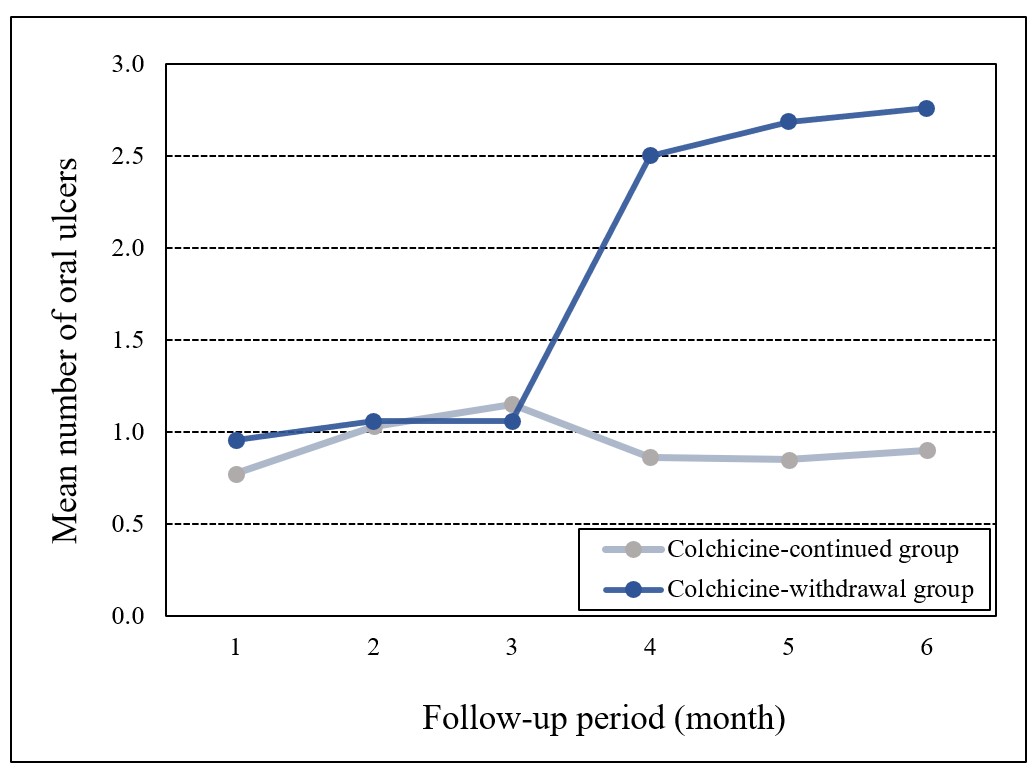
Results: A total of 141 patients were randomized to continue (70 patients, 45 F/25 M, mean age 45±10 years) or withdraw (71 patients, 46 F/25 M, mean age 46±11 years) colchicine (Figure 1). The mean number of oral ulcers at week 12 was higher in colchicine withdrawal group compared to patients who continued colchicine (2.7±2.6 vs 0.9±1.2, p< 0.001) (Figure 2). This significant difference was observed among both patients who were active at randomization (4.3±2.6 vs 1.4±1.2, p< 0.001), and among patients who had minimal disease activity at randomization (1.1±1.7 vs 0.5±1.0, p=0.046). Secondary endpoints including the number of genital ulcers, erythema nodosum-like lesions and papulopustular lesions, pain of oral and genital ulcers, and overall disease activity assessed by BDCAF and BSAS scores were higher among patients who discontinued colchicine (Table 1). On the other hand, there was no difference in BDQoL scores between patients who withdrew and who continued colchicine. Major organ manifestations were observed in 4 patients in the colchicine withdrawal group. These were deep vein thrombosis in 2 patients, myocarditis in 1 patient, and peripheral artery disease in 1 patient.
Conclusion: The significantly higher number of oral ulcers in patients who discontinued colchicine suggests that colchicine is effective for oral ulcers in some patients with BS. The significantly higher number of other mucocutaneous lesions and arthritis in patients who discontinued colchicine supports its efficacy in managing mucocutaneous and joint involvement of BS.
To cite this abstract in AMA style:
Sirin B, Esatoglu S, Yazıcı H, Hatemi G. A Withdrawal Study of Colchicine in Behçet Syndrome [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/a-withdrawal-study-of-colchicine-in-behcet-syndrome/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-withdrawal-study-of-colchicine-in-behcet-syndrome/