Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: To compare effectiveness, safety and costs of standard versus individually tailored reduced doses of anti-TNF drugs in patients with Ankylosing Spondylitis (AS) after achieving low disease activity.
Methods: This was a single center prospective observational study performed within the national biologics registry. The anti-TNF dose tapering strategy was chosen by treating physicians, without pre-specified protocol. We used propensity score (PS) methodology to identify 2 cohorts of patients matched for relevant baseline characteristics (table 1) who were treated with either reduced (n=53) or standard (n=83) doses of TNF inhibitors. One year outcomes and costs of anti-TNF drugs were compared between both PS-matched cohorts.
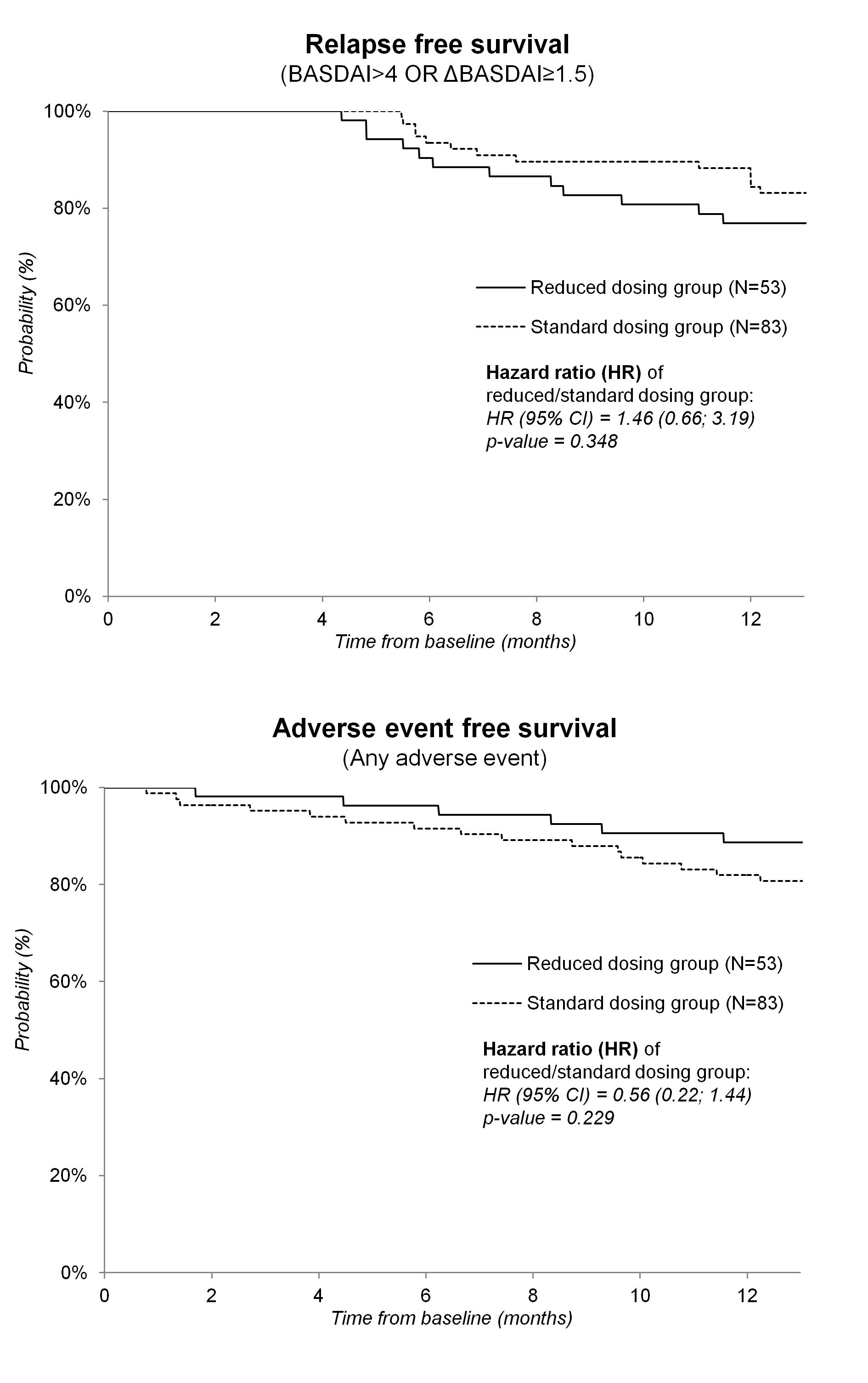
Results: In the reduced dosing group the median dose of TNF inhibitor corresponded to 0.67, and 0.5 of the standard dose initially, and at 12 months resp., and 21% of patients required return to standard dosing regimen. The mean change per year in BASDAI, CRP, HAQ and BASFI, as well as QALY area under the curve were no different between both groups (table 2). The hazard ratio (95% confidence interval) of reduced versus standard dosing group for relapse and any adverse event was 1.46 (0.66; 3.19), and 0.56 (0.22; 1.44) resp. (Figure 1) Mean difference (95% confidence interval) in cost of anti-TNF drugs was -4214 (-4707; -3701) € per year of treatment in favor of reduced dosing strategy.
Conclusion: In AS patients after reaching low disease activity, a tailored approach to reduce doses of anti-TNF drugs produced similar clinical outcomes at 1 year, but was substantially less costly.
Acknowledgements: This work was supported by project of MHCR for conceptual development of research organization 023728
Table 1 Baseline characteristics
|
|
|
Standard dosing group |
Reduced dosing group |
p-value |
|
|
n=83 |
n=53 |
|
|
|
Female |
n (%) |
19 (22.9 %) |
13 (24.5 %) |
0.840 |
|
Age (years) |
Mean (SD) |
39.5 (9.3) |
41.0 (10.8) |
0.585 |
|
Weight (kg) |
Mean (SD) |
75.3 (13.5) |
75.5 (14.9) |
0.927 |
|
HLA B27 positive |
n (%) |
76 (91.6 %) |
48 (90.6 %) |
0.919 |
|
Disease duration prior to the start of anti-TNF therapy (years) |
Mean (SD) |
8.1 (7.0) |
9.2 (8.5) |
0.582 |
|
Duration of anti-TNF therapy (months) |
Mean (SD) |
37.4 (20.0) |
34.8 (18.6) |
0.443 |
|
Peripheral joint involvement |
n (%) |
29 (34.9 %) |
18 (34.0 %) |
0.911 |
|
CRP (mg/l) |
Mean (SD) |
4.4 (5.6) |
4.3 (7.8) |
0.713 |
|
BASDAI |
Mean (SD) |
1.4 (0.9) |
1.4 (1.1) |
0.796 |
|
HAQ |
Mean (SD) |
0.4 (0.5) |
0.4 (0.4) |
0.833 |
|
BASFI |
Mean (SD) |
1.9 (1.4) |
1.8 (1.5) |
0.644 |
|
Concomitant glucocorticoids |
n (%) |
4 (4.8 %) |
1 (1.9 %) |
0.460 |
|
Concomitant DMARD |
n (%) |
9 (10.8 %) |
6 (11.3 %) |
0.954 |
|
Anti-TNF agents |
Etanercept, n (%) |
31 (37.3 %) |
25 (47.2 %) |
0.515 |
|
|
Adalumumab, n (%) |
19 (22.9 %) |
11 (20.8 %) |
|
|
|
Infiliximab, n (%) |
33 (39.8 %) |
17 (32.1 %) |
|
|
First anti-TNF treatment |
n (%) |
68 (81.9 %) |
46 (86.8 %) |
0.466 |
|
Mann-Whitney U test and unconditional z-pooled test are used when comparing continuous and categorical variables, respectively (Fisherxs exact test is used when comparing anti-TNF agents). |
||||
Table 2 Measures of activity/function, quality of life, and costs of anti-TNF therapy over one year of observation
|
|
|
Standard dosing group |
Reduced dosing group |
p-value |
|
|
|
n=83 |
n=53 |
|
|
BASDAI at baseline |
Mean (SD) |
1.4 (1.0) |
1.4 (1.1) |
0.796 |
|
BASDAI at 12 M |
Mean (SD) |
1.9 (1.5) |
1.7 (1.3) |
0.453 |
|
Change in BASDAI (per year) |
Mean (95% CI) |
0.47 (0.18; 0.76) |
0.36 (0.01; 0.71) |
0.615 |
|
Difference of mean change (per year) |
Mean (95% CI) |
reference |
-0.12 (-0.57; 0.34) |
|
|
CRP at baseline |
Mean (SD) |
4.4 (5.9) |
4.3 (7.9) |
0.713 |
|
CRP at 12 M |
Mean (SD) |
7.5 (15.6) |
5.4 (7.9) |
0.992 |
|
Change in CRP (per year) |
Mean (95% CI) |
3.42 (-0.16; 7.01) |
2.19 (-2.09; 6.47) |
0.663 |
|
Difference of mean change (per year) |
Mean (95% CI) |
reference |
-1.23 (-6.81; 4.35) |
|
|
HAQ at baseline |
Mean (SD) |
0.4 (0.4) |
0.4 (0.4) |
0.833 |
|
HAQ at 12 M |
Mean (SD) |
0.4 (0.4) |
0.4 (0.5) |
0.479 |
|
Change in HAQ (per year) |
Mean (95% CI) |
0.07 (0.00; 0.14) |
0.08 (-0.01; 0.17) |
0.942 |
|
Difference of mean change (per year) |
Mean (95% CI) |
reference |
0.00 (-0.11; 0.12) |
|
|
BASFI at baseline |
Mean (SD) |
1.9 (1.7) |
1.8 (1.7) |
0.644 |
|
BASFI at 12 M |
Mean (SD) |
2.1 (1.8) |
1.9 (1.7) |
0.481 |
|
Change in BASFI (per year) |
Mean (95% CI) |
0.07 (-0.21; 0.35) |
0.09 (-0.24; 0.43) |
0.907 |
|
Difference of mean change (per year) |
Mean (95% CI) |
reference |
0.03 (-0.41; 0.46) |
|
|
EQ-5D* utility at baseline |
Mean (SD) |
0.80 (0.09) |
0.79 (0.11) |
0.667 |
|
EQ-5D* utility at 12 months |
Mean (SD) |
0.78 (0.14) |
0.78 (0.11) |
0.901 |
|
QALY area under the curve** |
Mean (SD) |
0.78 (0.12) |
0.76 (0.14) |
0.436 |
|
Annual cost of anti-TNF therapy (€) |
Mean (SD) |
12 000 (-) |
7 784 (2 254) |
<0.001 |
|
Incremental effectiveness*** |
Mean (95% CI) |
reference |
-0.020 (-0.057; 0.016) |
|
|
Incremental cost (€)*** |
Mean (95% CI) |
reference |
-4 214 (-4 707; -3 701) |
|
|
Mann-Whitney U test was used when comparing continuous variables. Change (per year) was estimated using linear mixed effects regression model and restricted maximum likelihood method. * EQ-5D utility was derived from BASDAI and BASFI. ** QALY was calculated as area under the curve of linearly interpolated values of EQ-5D utility. ***Incremental cost and effectiveness are differences between groups estimated from 10000 bootstrap samples. |
||||
Figure 1
Disclosure:
J. Zavada,
None;
M. Uher,
None;
K. Sisol,
None;
S. Forejtova,
None;
K. Jarosova,
None;
H. F. Mann,
None;
J. Vencovsky,
None;
K. Pavelka,
None.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-tailored-approach-to-reduce-dose-of-anti-tnf-drugs-is-equally-effective-but-substantially-less-costly-than-standard-dosing-in-patients-with-ankylosing-spondylitis-over-one-year-a-propensity-score/