Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Cost is a major impediment for use of biologic response modifiers (BRMs). Biosimilars/Intended copies, priced at almost half the reference molecule might be the answer. There is limited data on safety & efficacy of these in pediatric rheumatology. This study was thus undertaken to i.) Assess efficacy/safety of Cipla Etanercept(CE) &Intas Etanercept(IE) ii.)Compare efficacy of CE& IE with reference Etanercept & with each other
Methods: All children with JIA who were given CE & IE till 15.6.16 were included. CE/IE/Reference Etanercept was used in disease resistant to Methotrexate, NSAIDs & steroids. Age matched 39 children who received reference Etanercept were taken as control arm. Data was analysed by Wilcoxon Signed Ranks test at 0, 3 & 6 mths for: 74 joint count, joints with limited range of movt., ESR & CRP. Patients were also categorised as per Wallace criteria into active disease, inactive disease & clinical remission on & off medications.
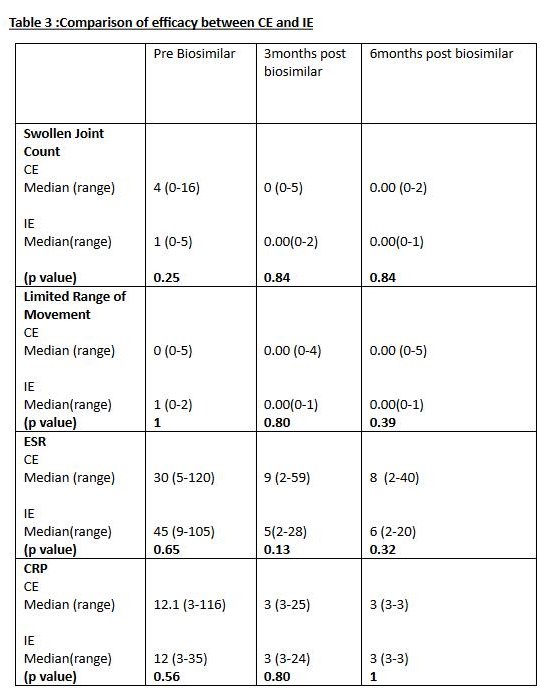
Results: Total 69 children – 30 given CE & IE, 39 given reference Etanercept. Demographics –CE & IE given to 30(53% males).ERA: 60%,Polyarticular JIA:30% & others 10%. Median age of starting CE & IE: 8.41yrs. Reference Etanercept given to 39(61%males).ERA:66.6%, Polyarticular JIA:20% &13.4% others. Median age at starting :10.66 yrs Efficacy– Table 1 shows significant efficacy of CE & IE in all tested domains. Table 2 shows no difference in all tested domains of reference Etanercept when compared to CE &IE.Table 3 shows no significant difference in efficacy of CE vs IE.
Safety: CE/IE were safe with few side effects. Pre CE/ IE: HbsAg +ve:1; Screening for tuberculosis(TB) infection +ve in 5 Pre Reference Etanercept – Screening for TB +ve in 3 Post CE & IE: 12 weeks after starting IE, 1(3.33%) had cellulitis of the right foot. Reference Etanercept: 3 (Hemolytic anemia-1,Varicella-1,New onset uveitis-1).
Conclusion: CE & IE licensed for use in India are safe &effective in a cohort of 30 children with JIA. They are comparable to each other & the reference Etanercept with a similar efficacy& safety profile. This is the first comparative study of these two agents against reference Etanercept from a single centre & needs to be replicated in other centres with a longer follow up.
To cite this abstract in AMA style:
Shivpuri A, Mittal S, Agarwal M, Sawhney S. A Single Centre Experience from India on the Safety and Efficacy of Cipla Etanercept and Intas Etanercept and Its Comparison with Reference Etanercept(Enbrel) in Children with JIA [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/a-single-centre-experience-from-india-on-the-safety-and-efficacy-of-cipla-etanercept-and-intas-etanercept-and-its-comparison-with-reference-etanerceptenbrel-in-children-with-jia/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-single-centre-experience-from-india-on-the-safety-and-efficacy-of-cipla-etanercept-and-intas-etanercept-and-its-comparison-with-reference-etanerceptenbrel-in-children-with-jia/