Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Data regarding the safety and efficacy of treatment regimens for juvenile dermatomyositis(JDM) tends to be from anecdotal, small, uncontrolled, non-randomized case series. This randomized trial was aimed to find out the treatment regimen associated with the lowest occurrence of flare and the lowest drug related toxicity.
Methods: Children with newly diagnosed JDM were randomized in an open fashion to receive one of 3 different therapeutic approaches:prednisone (PDN) versus PDN plus methotrexate (MTX) versus PDN plus Cyclosporine A.The overall hypothesis to be tested in this trial was that the early introduction of combination therapy of corticosteroids and either MTX or CsA will prove more effective and safe than corticosteroids alone in the treatment of JDM.
Primary outcome measures after 6 months of treatment: response rate according to the PRINTO provisional definition of improvement in the 3 arms (20% improvement in at least 3 core set variables with no more than 1 of the remaining variables, (muscle strength excluded), worsened by > 30%).The PRINTO JDM core set variables are: 1)muscle strength by the mean of the Childhood Myositis Assessment Scale (CMAS); 2)physician’s global assessment of disease activity on a 10 cm VAS ;3)global disease activity assessment by the mean of the Disease Activity Index (DAS); 4)parent’s/patient’s global assessment of overall well-being on a 10 cm VAS; 5)functional ability assessment by the mean of the Childhood Health Assessment Questionnaire (CHAQ); 6)health-related quality of life assessment.
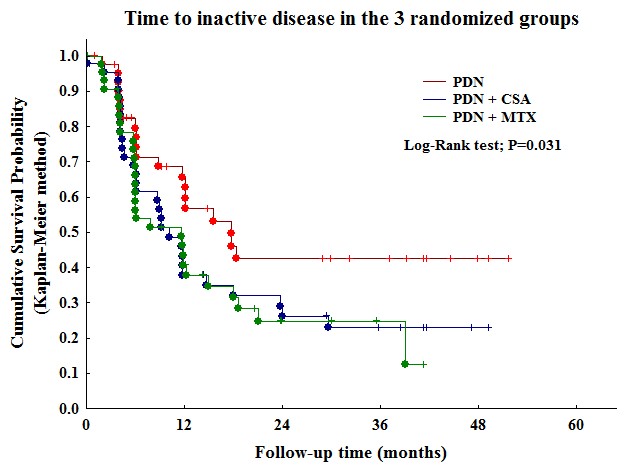
Primary outcome measures after 24 months of treatment: a) time to inactive disease; b) time to major therapeutic changes because of inefficacy/flare/adverse events.
Results: 138/139 randomized patients were included in the efficacy dataset. There were 81/138 females (59%) with a median age at onset of 7.4 years (1st-3rd quartiles 1.1-15.4) and a median disease duration of 2.8 months (1.4-5.3). Frequency of response at 6 months was for 24/46 (52.2%) for PDN, 31/46 (67.4%) for PDN+CSA and 34/46 (73.9%) for PDN+MTX (p 0.082). Time to inactive disease (Figure) in the combination group (PDN+CSA or PDN+MTX) was significantly shorter than that of PDN alone (p 0.031). Time to major therapeutic changes in the combination group (PDN+CSA or PDN+MTX) was significantly longer than that of PDN alone (p 0.006). Total number of adverse events were 57/276 (20.7%) in the PDN group, 141/276 (51.1%) in PDN+CSA and 78//276 (28.3%) in PDN+MTX (p < 0.0001). Skin and subcutaneous tissue disorders, and nervous system disorders were statistically more frequent in PDN+CSA (p 0.0015, p 0.039 respectively).
Conclusion: combined therapy with PDN and either CSA or MTX was more effective than with PDN alone.However the safety profile favour the combination with MTX toward that with CSA.
http://acr.confex.com/acr/2012/acr/papers/index.cgi?username=31116&password=628996
Disclosure:
N. Ruperto,
None;
A. Pistorio,
None;
S. Oliveira,
None;
R. J. Cuttica,
None;
A. Ravelli,
None;
M. Fischbach,
None;
S. Hagelberg,
None;
T. Avcin,
None;
E. Cheuret,
None;
F. Corona,
None;
G. Couillault,
None;
F. Dressler,
None;
V. Gerloni,
None;
G. Sterba Sr.,
None;
F. Zulian,
None;
M. T. Apaz,
None;
A. Cespedes-Cruz,
None;
R. Cimaz,
None;
F. De Benedetti,
None;
P. Quartier,
None;
R. Russo,
None;
N. Wulffraat,
None;
S. Angioloni,
None;
A. Martini,
None.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-randomized-trial-in-new-onset-juvenile-dermatomyositis-prednisone-versus-prednisone-plus-cyclosporine-versus-prednisone-plus-methotrexate/