Session Information
Session Type: Abstract Submissions (ACR)
A Randomized Placebo-Controlled Phase 2 Study to Evaluate Efficacy, Safety and Tolerability of Two Secukinumab Loading Dose Regimens in Subjects with Active Rheumatoid Arthritis Despite Treatment with Methotrexate
Background/Purpose: To evaluate the efficacy, safety and tolerability at week 12 of two loading regimens of secukinumab (AIN457), a fully human anti-interleukin-17A monoclonal antibody, compared to placebo and to determine whether i.v. and s.c. loading doses of secukinumab offer comparable results for patients with active RA despite treatment with methotrexate.
Methods: This was a randomized, double-blind, 12-week, phase 2 study in subjects with active RA despite stable methotrexate treatment. Subjects were randomized (2:2:1) to receive secukinumab i.v. loading (10 mg/kg week 0, 2 and 4, followed by 150 mg s.c. week 8), secukinumab s.c. loading (150 mg week 0, 1, 2, 3, 4 and 8) or placebo. The primary objective was to demonstrate the superior efficacy of pooled secukinumab compared to placebo using ACR20 criteria at week 12. Secondary efficacy objectives included ACR20/50/70, ACR components and DAS28 for pooled and individual secukinumab treatment arms.
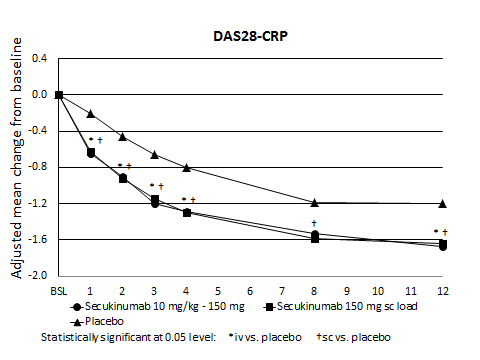
Results: A total of 221 subjects were randomized: 88 to i.v. loading, 89 to s.c. loading and 44 to placebo. All subjects were included in efficacy and safety analyses. Subjects were mainly from Eastern Europe (78%) and had a median of 12 swollen and 20 tender joints at baseline. All were positive for either anti-CCP or RF, with a CRP ≥ 10 mg/L or ESR ≥ 28 mm/1st hr. ACR20 response rate at week 12 was numerically but not statistically significantly greater with pooled secukinumab (49.2%) compared to placebo (40.9%). Reductions of DAS28-CRP were significantly greater for both secukinumab arms compared to placebo at all visits (except i.v. loading week 8) (Figure). No statistically significant differences in ACR20, DAS28-CRP or other efficacy outcomes were observed between the i.v. and s.c. loading regimens. The safety profile was similar to that in previous secukinumab RA trials. The proportion of pooled secukinumab-treated subjects vs. placebo-treated subjects was 41.2% vs. 38.6% for any AE, 2.8% vs. 2.3% for serious AEs, and 1.7% vs. 0% for AEs leading to discontinuation.
Conclusion: Secukinumab demonstrated improved efficacy in reducing disease activity over placebo as measured by DAS28-CRP, but not ACR20 at week 12 (primary endpoint). Reductions in disease activity were very similar for the i.v. and s.c. secukinumab loading regimens. The AE profile of secukinumab was comparable to placebo. Phase 3 studies in RA patients with inadequate response to anti-TNF treatments are ongoing.
Figure. DAS28-CRP response rates by treatment arm through week 12
Disclosure:
S. Adami,
MSD, Amgen, Novartis,
5;
A. Beaulieu,
None;
P. Rahman,
Amgen, Abbott, BMS, Merck, Pfizer, Janssen, Hoffman-La Roche, UCB, Novartis, Sanofi-Aventis,
5,
Amgen, Abbott, BMS, Merck, Pfizer, Janssen, Hoffman-La Roche, UCB, Novartis, Sanofi-Aventis,
9;
B. Seriolo,
None;
J. S. Lee,
Novartis Pharma AG,
3;
G. Krammer,
Novartis,
1,
Novartis ,
3;
B. Porter,
Novartis Pharma AG,
1,
Novartis Phama AG,
3,
Primary Care: The Art and Science of Advanced Practice Nursing FA Davis),
7;
A. Thulasiraman,
None;
H. B. Richards,
Novartis Pharma AG,
3.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-randomized-placebo-controlled-phase-2-study-to-evaluate-efficacy-safety-and-tolerability-of-two-secukinumab-loading-dose-regimens-in-subjects-with-active-rheumatoid-arthritis-despite-treatment-with/