Session Information
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Takayasu’s arteritis (TAK) is a large-vessel primary systemic vasculitis that affects the aorta, its branches, and the pulmonary arteries. Despite treatment with glucocorticoids, relapse with treatment- and disease-related morbidity occurs in a high percentage of patients. Several other immunosuppressive agents have been used to treat TAK, but their use is based solely on open-label experience. T-cell activation has been implicated in the pathophysiology of TAK. A multi-center, randomized, double-blind, placebo-controlled, withdrawal design trial was conducted to examine the efficacy and safety of treatment with abatacept (CTLA4-Ig) combined with prednisone in patients with TAK.
Methods: Patients with newly-diagnosed or relapsing TAK were eligible for the trial. All patients were treated with abatacept 10 mg/kg IV on days 1, 15, 29, and week 8, together with prednisone. At week 12 patients in remission underwent a double-blinded randomization to continue monthly abatacept or be switched to placebo together with a standardized prednisone taper reaching discontinuation at week 28. Patients remained on their randomized assignment until meeting criteria for early termination or until the common closeout date, 12 months after enrollment of the last patient. The primary endpoint was duration of remission (relapse-free survival, RFS). A planned sample size of 30 patients was based on detecting a 30% improvement in RFS utilizing a one-sided alpha = 0.1. Kaplan-Meier curves were constructed and differences in treatment arms compared using the log-rank test in an intent-to-treat analysis.
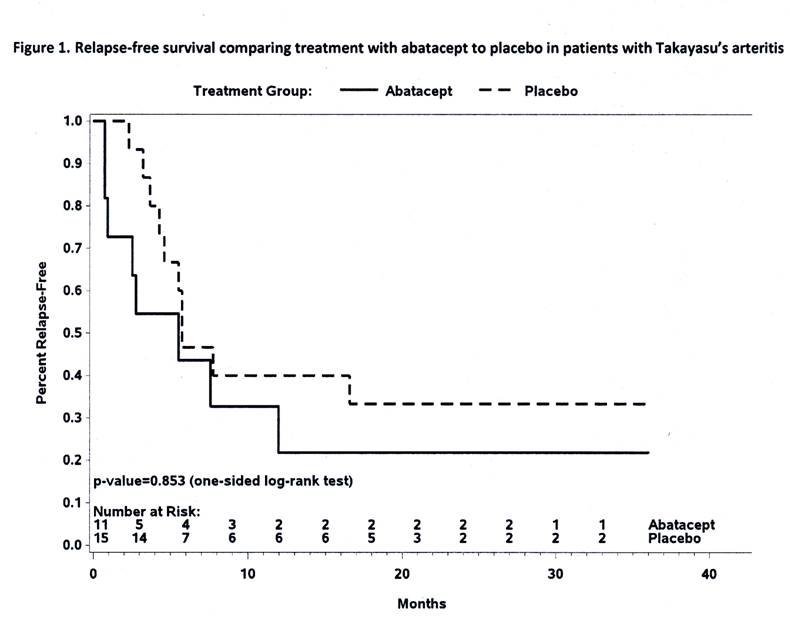
Results: 34 eligible patients received study drug with 26 reaching the week 12 randomization. Disease characteristics of the 26 randomized patients are outlined in Table 1. The RFS at 12 months was estimated to be 22% for those receiving abatacept and 40% for those receiving placebo (p= 0.853), Figure 1. Treatment with abatacept in patients with TAK enrolled in this study was not associated with a longer median duration of remission (abatacept 5.5 months, placebo 5.7 months). 114 adverse events occurred in 28 patients including 23 serious adverse events in 15 patients. There was no difference in the frequency or severity of adverse events between treatment arms, including the rate of infection.
Conclusion: In this study, which was the first randomized, double-blind trial conducted in TAK, the addition of abatacept to prednisone did not reduce the risk of relapse in patients with TAK. Concurrent abatacept was not associated with a higher rate of toxicity compared to prednisone alone.
To cite this abstract in AMA style:
Langford CA, Cuthbertson D, Ytterberg SR, Khalidi NA, Monach PA, Carette S, Seo P, Moreland LW, Weisman M, Koening CL, Sreih AG, Spiera RF, McAlear CA, Warrington KJ, Pagnoux C, Maksimowicz-McKinnon K, Forbess LJ, Hoffman GS, Borchin R, Krischer J, Merkel PA. A Randomized Double-Blind Trial of Abatacept and Glucocorticoids for the Treatment of Takayasu’s Arteritis [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/a-randomized-double-blind-trial-of-abatacept-and-glucocorticoids-for-the-treatment-of-takayasus-arteritis/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-randomized-double-blind-trial-of-abatacept-and-glucocorticoids-for-the-treatment-of-takayasus-arteritis/