Session Information
Date: Tuesday, November 14, 2023
Title: (1996–2018) Osteoporosis & Metabolic Bone Disease – Basic & Clinical Science Poster
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: SB16 is a human monoclonal antibody to the receptor activator of nuclear factor κΒ ligand that has been developed as a proposed biosimilar to reference denosumab (brand name: Prolia, DEN hereafter). This Phase III study was a randomized double-blind study to compare efficacy, safety, pharmacokinetics (PK), pharmacodynamics (PD) and immunogenicity of SB16 with DEN in postmenopausal osteoporosis (PMO) patients (NCT04664959). Results up to Month 12 are presented here.
Methods: PMO patients were randomized in a 1:1 ratio to receive either 60 mg of SB16 or DEN subcutaneously at Month 0, Month 6, and Month 12. At Month 12, patients in DEN were re-randomized in a 1:1 ratio to switch to SB16 or maintain DEN. The primary endpoint was percent (%) change from baseline in lumbar spine bone mineral density (BMD) at Month 12. Equivalence between SB16 and DEN was declared if the 95% confidence interval (CI) of Least Squares Means (LSMeans) difference of % change in lumbar spine BMD at Month 12 was within the pre-defined equivalence margin. Other secondary efficacy, PD (serum C-telopeptide of type I collagen [CTX] and procollagen type I N-terminal propeptide [P1NP]), PK and safety endpoints were also measured.
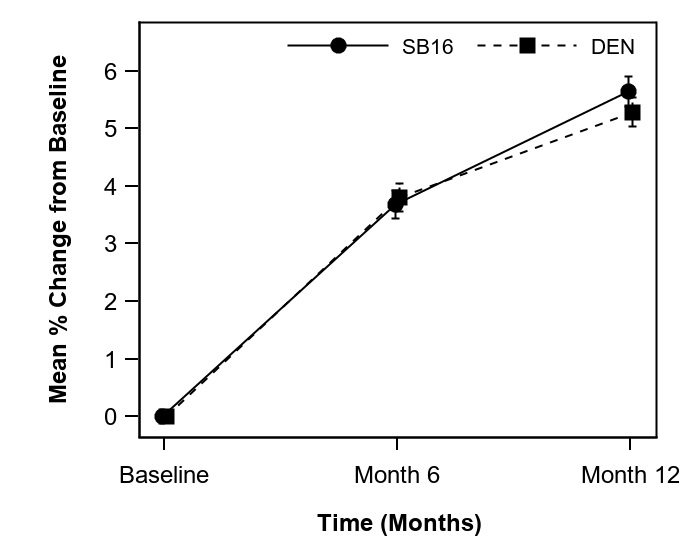
Results: Among 457 patients, 225 patients were randomized to SB16 and 232 patients to DEN. Baseline characteristics were comparable between SB16 and DEN. The mean % change from baseline in lumbar spine BMD at Month 12 was5.6% and 5.3% in SB16 and DEN, respectively (Figure 1), and the difference in LSMeans % was 0.39, 95%CI [−0.36, 1.13] for the per-protocol set, and 0.33, 90%CI [−0.25, 0.91] for the full analysis set, both within the pre-defined equivalence margin. The mean % change from baseline in total hip BMD at Month 12 was 3.5% and 3.2% in SB16 and DEN, respectively. The mean % change from baseline in femoral neck BMD at Month 12 was 2.8% and 2.3% in SB16 and DEN, respectively. The incidence and distribution of adverse events were comparable between SB16 and DEN up to Month 12. Serum CTX, P1NP and denosumab concentrations were comparable between SB16 and DEN up to Month 12. The median % change from baseline in serum CTX concentration over time was a decrease of at least 50% at all time points. Only 3 subjects (1 in SB16 and 2 in DEN) had non-neutralizing anti-drug antibodies.
Conclusion: This study demonstrated biosimilarity of SB16 to DEN through equivalent efficacy and comparable PD, PK, immunogenicity and safety up to Month 12.
To cite this abstract in AMA style:
Eastell R, Langdahl B, Chung Y, Plebanski R, Czerwinski E, Dokoupilova E, Supronik J, Rosa J, Rowińska-Osuch A, Baek K, Urboniene A, Ahn S, Rho Y, Ban J. A Randomized, Double-blind, Phase III Study to Compare SB16 (Proposed Denosumab Biosimilar) to Reference Denosumab in Patients with Postmenopausal Osteoporosis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/a-randomized-double-blind-phase-iii-study-to-compare-sb16-proposed-denosumab-biosimilar-to-reference-denosumab-in-patients-with-postmenopausal-osteoporosis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-randomized-double-blind-phase-iii-study-to-compare-sb16-proposed-denosumab-biosimilar-to-reference-denosumab-in-patients-with-postmenopausal-osteoporosis/