Session Information
Date: Sunday, November 10, 2019
Title: 3S032: Plenary I (805–809)
Session Type: Plenary Session I
Session Time: 11:00AM-12:30PM
Background/Purpose: Rituximab is an effective therapy for induction of remission in ANCA-associated vasculitis (AAV). However, the effect of rituximab is not sustained, and relapse rates are high, especially in patients with a history of relapse. The RITAZAREM trial (ClinicalTrials.gov identifier: NCT01697267) is an international, multi-center, open-labelled, randomized, controlled trial of patients with AAV with relapsing disease comparing the efficacy, after induction of remission with rituximab, of two relapse-prevention strategies: repeat dose rituximab or daily oral azathioprine.
Methods: Patients with AAV were recruited at time of relapse and received induction therapy with rituximab and glucocorticoids. If remission was achieved by month 4, patients were randomized in a 1:1 ratio to receive either rituximab (1000 mg every 4 months for 5 doses) or azathioprine (2 mg/kg/day) as maintenance therapy. Patients were followed for a minimum of 36 months, with the primary outcome being time to disease relapse. The formal hypothesis testing plan initially considers the hazard ratio for relapse across all time periods. If, and only if this global test is significant at a 5% level then the hazard ratios during the treatment period and the follow-up periods are considered separately.
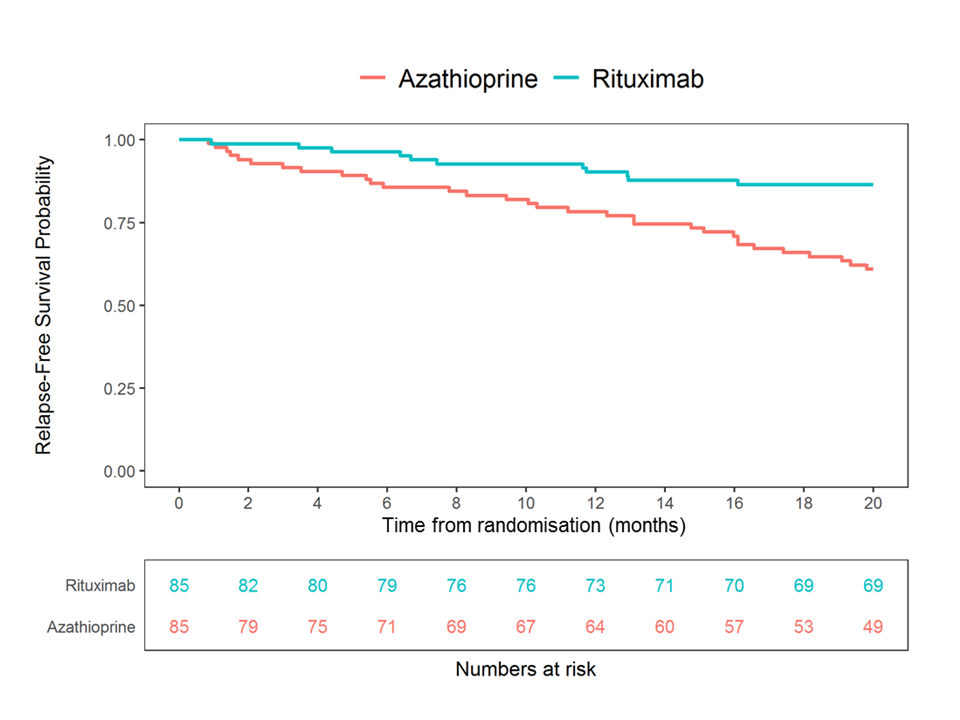
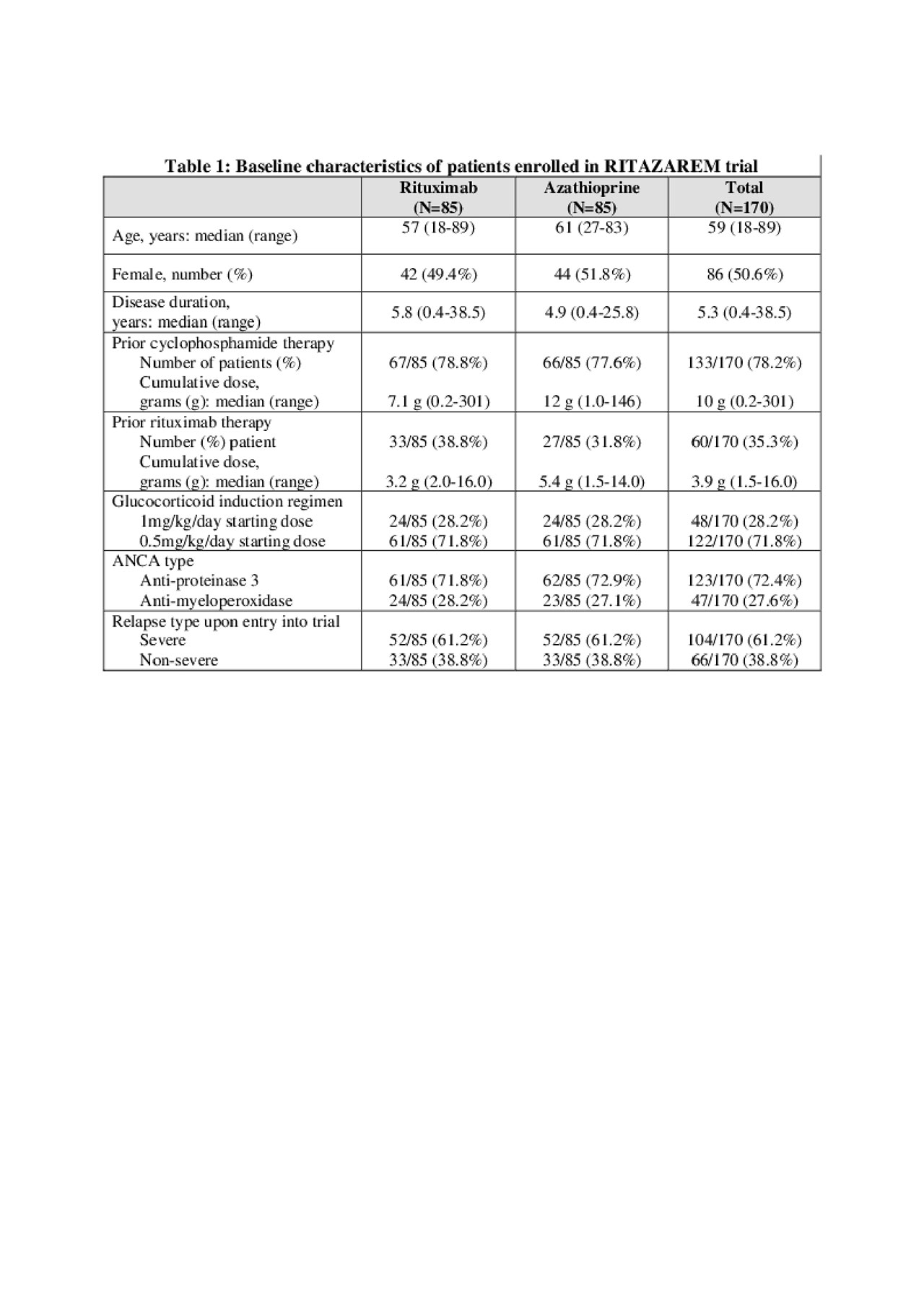
Results: 190 patients were enrolled and 170 randomized at 4 months (85 to rituximab; 85 to azathioprine). The data are complete on all patients up to at least month 24. Median age was 59 years (range 19-89), with a prior disease duration of 5.3 years (0.4-38.5). 123/170 (72%) patients had a history of testing positive for anti-proteinase 3 ANCA; 47/170 (28%) for myeloperoxidase ANCA; 104/170 (61%) were enrolled having suffered a major relapse, and 48/170 (28%) received a pre-specified higher dose glucocorticoid induction regimen (Table 1). Rituximab was superior to azathioprine in preventing disease relapse with a preliminary overall hazard ratio (HR) estimate of 0.36 (95% CI 0.23-0.57, p < 0.001) and a during-treatment HR estimate of 0.30 (95% CI 0.15-0.60, p< 0.001) (Figure 1). After adjustment, none of the randomization stratification covariates (ANCA type, glucocorticoid induction regimen, or relapse severity) had a significant differential effect on the primary outcome. By 24 months after entry, 20 months after randomization, 11/85 (13%) patients in the rituximab group had experienced a relapse compared to 32/85 (38%) patients in the azathioprine group. In the rituximab group 2/11 (18%) relapses were classified as major, compared to 12/32 (38%) in the azathioprine group. 19/85 (22%) patients in the rituximab group and 31/85 (36%) patients in the azathioprine group experienced at least one severe adverse event (SAE). 25/85 (29%) and 42/85 (49%) patients in the rituximab group developed hypogammaglobulinaemia (IgG < 5g/l) and non-severe infections respectively, compared to 21/85 (25%) and 41/85 (48%) in the azathioprine group. Conclusion: In the RITAZAREM trial, following induction of remission with rituximab, rituximab was superior to azathioprine for preventing disease relapse in patients with AAV with a prior history of relapse. There were no new major safety signals for use of these medications in this population.
RITAZAREM ACR Abstract _table1
To cite this abstract in AMA style:
Smith R, Jayne D, Merkel P. A Randomized, Controlled Trial of Rituximab versus Azathioprine After Induction of Remission with Rituximab for Patients with ANCA-associated Vasculitis and Relapsing Disease [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/a-randomized-controlled-trial-of-rituximab-versus-azathioprine-after-induction-of-remission-with-rituximab-for-patients-with-anca-associated-vasculitis-and-relapsing-disease/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-randomized-controlled-trial-of-rituximab-versus-azathioprine-after-induction-of-remission-with-rituximab-for-patients-with-anca-associated-vasculitis-and-relapsing-disease/