Session Information
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Immune checkpoint inhibitors (ICIs) are increasingly becoming the mainstay of management of advanced malignancies, but can result in immune related adverse events (irAEs) affecting nearly every organ system. The purpose of this retrospective study is to determine the incidence and nature of irAEs in patients treated with ICIs at our single center university hospital. The primary objective was to assess risk factors for development of irAEs. Secondary objectives included determining 1) incidence of rheumatic and non-rheumatic irAEs; 2) time to onset of irAEs; 3) proportion required to discontinue ICIs due to irAEs; 4) comparative risk of specific ICIs causing irAEs.
Methods: Patients over age 18 were identified by electronic chart review with oncologist-diagnosed cancer who were prescribed ICIs between March 31, 2011 to December 31, 2018. ICIs included cytotoxic T-lymphocyte-associated protein 4 inhibitors (CTLA-4), programmed death-1/programmed death-ligand 1 inhibitors (PD-1/PD L-1) or combination therapy. We performed a detailed chart review to collect patient demographics, type of cancer, ICI(s) utilized, time to onset of irAEs, type of irAEs, treatment, and outcome. Cases were defined as patients who either developed new irAEs or experienced a flare of their pre-existing autoimmune disease after initiation of ICI. Controls were defined as those without any irAEs. The Fisher test and the Kruskal-Wallis test were used to compare categorical and continuous variables, respectively.
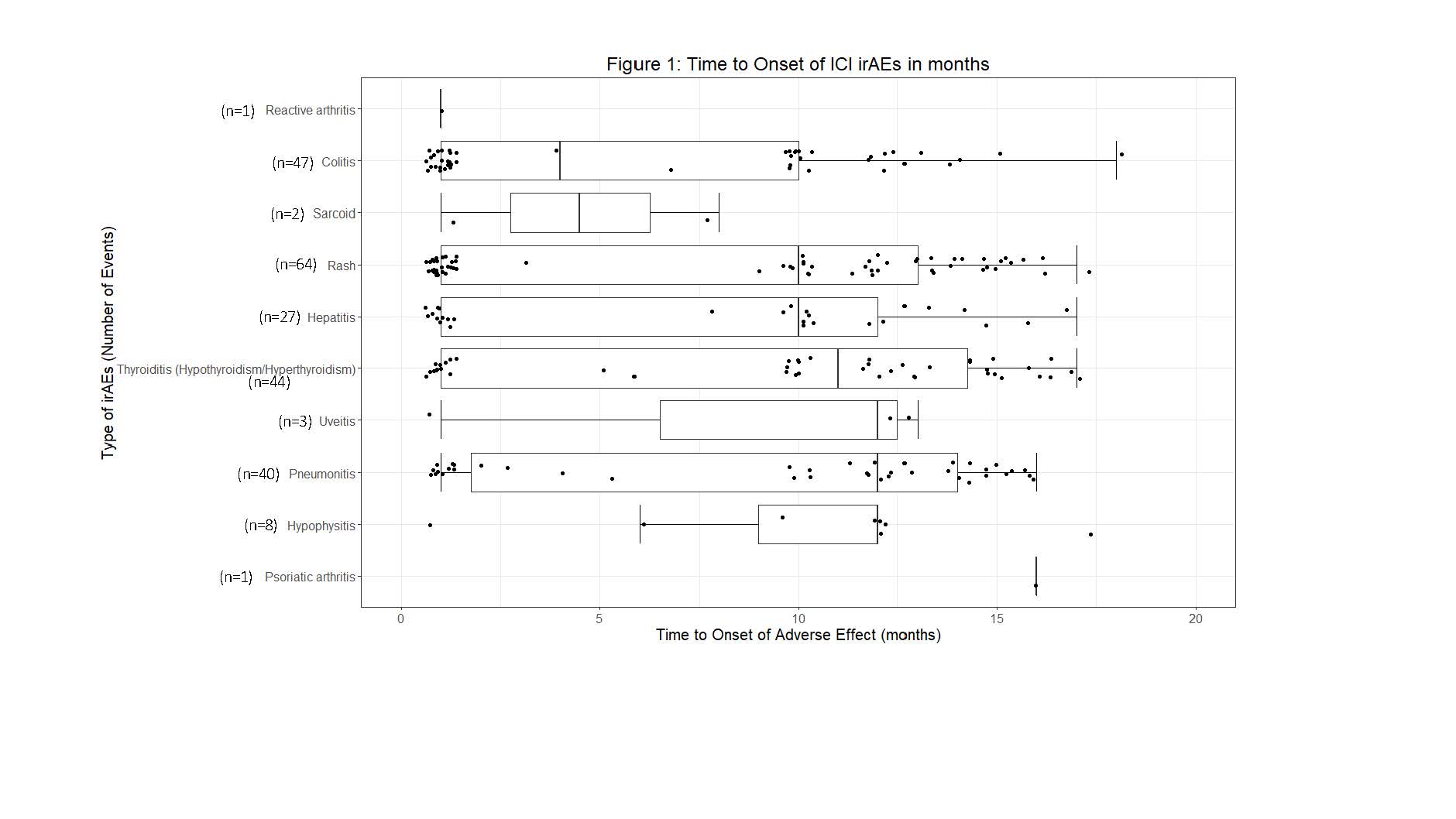
Results: A total of 827 patients were prescribed ICIs, of which 209 (25%) developed 264 independent irAEs. A comparison of those who developed irAEs and those who did not is shown in Table 1. Sixteen patients (6%) developed rheumatic irAEs. Seven patients developed inflammatory arthritis. Less common rheumatic irAEs included uveitis, myositis, psoriatic arthritis, reactive arthritis and sarcoidosis. The majority were treated with oral prednisone. Time to onset of select irAEs since initiation of ICIs is shown in Figure 1. Of the 209 with irAEs, 145 (69.4%) required temporary/permanent discontinuation of ICIs due to irAEs. The odds of developing irAEs were 3.6 times higher with CTLA-4 compared to PD-1/PD-L1 agents. Odds for developing irAEs were also 1.5 times higher with combination therapy than CTLA-4 monotherapy, and 5.2 times higher than PD-1/PD-L1 alone.
Conclusion: We found that a quarter of patients treated with ICIs developed irAEs, and that non-rheumatic irAEs were much more common than rheumatic irAEs. Type of malignancy, type of ICI agent and nature of previous cancer therapies factored into the development of irAEs. The odds of developing irAEs were higher with usage of combination ICI compared to the single agents. However, in our study no rheumatic irAEs were attributed to CTLA-4 use alone. This could be explained by sample size. Data continues to remain scarce on treatment and long-term follow up of rheumatic irAEs. Partnering with oncologists, expediting patient referral to a dedicated ICI-rheumatology clinic and establishing multicenter registries will provide more robust data on these heterogenic irAEs.
To cite this abstract in AMA style:
Zhou C, Elg-Salsman W, Kiwalkar S, Sathe N, Friedman M, Deodhar A. A Quarter of Patients Treated with Checkpoint Inhibitors Develop Immune-Related Adverse Events: A University Center Experience [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/a-quarter-of-patients-treated-with-checkpoint-inhibitors-develop-immune-related-adverse-events-a-university-center-experience/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-quarter-of-patients-treated-with-checkpoint-inhibitors-develop-immune-related-adverse-events-a-university-center-experience/