Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Hydroxychloroquine (HCQ) is a commonly used medication in our field of work with retinal toxicity being a known possible long-term side effect of the medication. Studies and case reports have described a role of HCQ in cardiotoxicity although, the relation is not completely clear [Drug Saf. 2018 Oct;41(10):919-931]. The gold standard of diagnosis would be an endomyocardial biopsy however, it is impractical to have the biopsy done on anyone suspected of having HCQ induced cardiomyopathy [J Rheumatol. 2019 Apr;46(4):391-396]. Our goal is to evaluate if cardiac injury markers as predictors of HCQ-induced cardiotoxicity.
Methods: This quality improvement project retrospectively analyzed medical record of patients taking HCQ with a diagnosis of an arrhythmia, cardiomyopathy, or heart failure at SUNY Upstate Medical University in Syracuse, New York. Established causes of heart disease, such as hypertension or congenital malformations were excluded. Patients were distinguished as to whether they had the cardiac diagnosis before or after the starting HCQ. Cardiac injury markers, CK, CKMB, troponin, BNP, were evaluated in each subject. Statistical analyses of categorical variables were performed with chi-square test using GraphPad software.
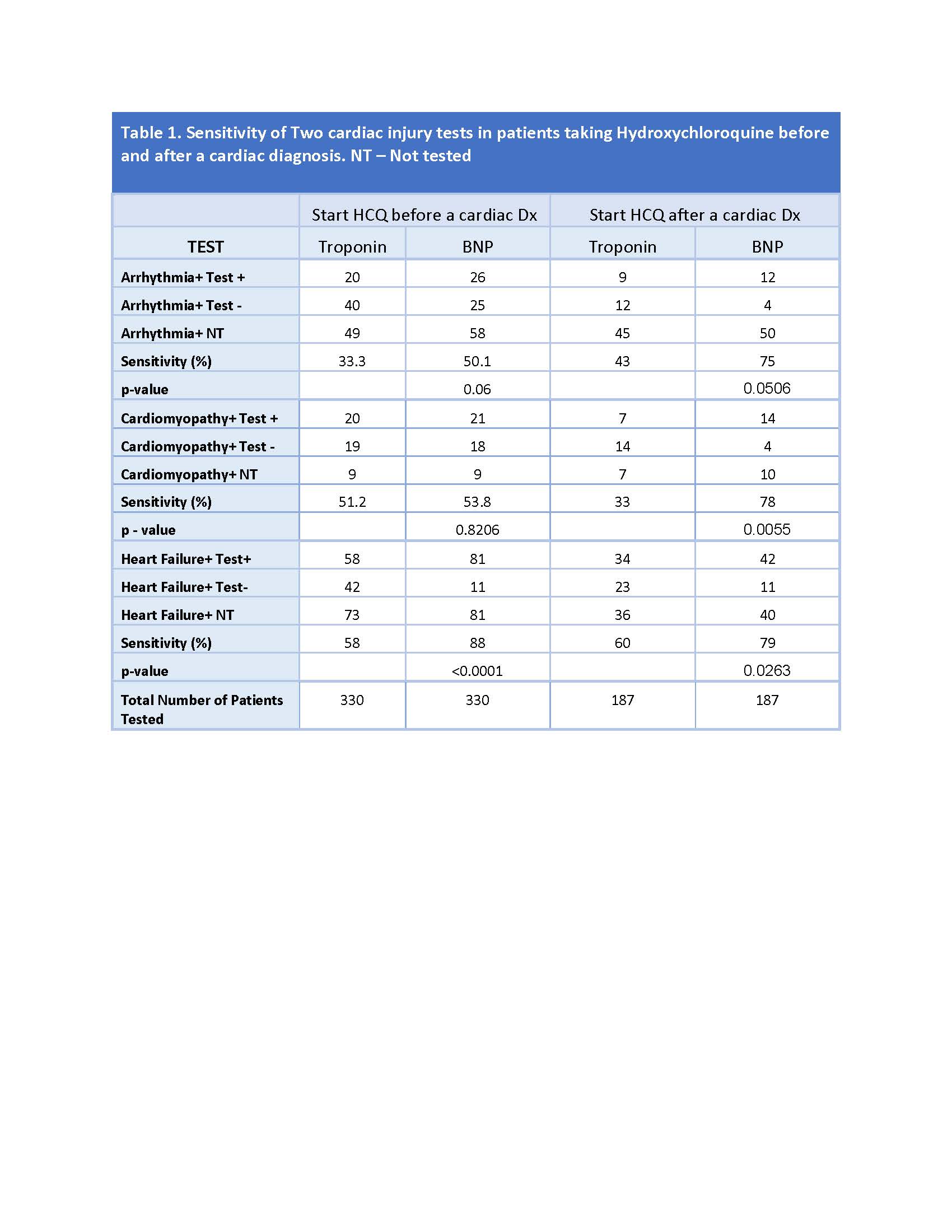
Results: 8220 patients were identified to be on HCQ between 2013 and 2019. 854/8220 (10.4%) had a cardiac diagnosis, such as arrhythmia, cardiomyopathy, or heart failure. 517/854 had a cardiac diagnosis not attributed to a known cause such as hypertension or congenital malformations. Of 517 cases, heart disease was recorded in 187 patients before and 330 patients after initiation of HCQ treatment. Among laboratory tests examined, BNP testing was found to be most sensitive to detect heart disease in both groups. In patients who were diagnosed with heart disease after having started HCQ, BNP was 88% sensitive for detection of heart failure (p< 0.0001 over other tests). BNP was also most sensitive to detect cardiomyopathy or heart failure in patients subsequently started on HCQ.
Conclusion: Cardiotoxicity is a newly recognized possible side effect of HCQ therapy. While clinicians are aware of surveillance for possible retinal and guidelines have been established, there are no guidelines for cardiotoxicity monitoring. This study indicates that BNP may be a sensitive test for detecting heart disease in patients taking HCQ either before or after initiation of therapy. These results advocate for including BNP for safety monitoring of patients treated with HCQ.
To cite this abstract in AMA style:
Liu E, Perl A. A Quality Improvement Project in Determining If Cardiac Enzymes Play a Role in Surveillance of Possible Hydroxychloroquine Induced Cardiotoxicity [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/a-quality-improvement-project-in-determining-if-cardiac-enzymes-play-a-role-in-surveillance-of-possible-hydroxychloroquine-induced-cardiotoxicity/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-quality-improvement-project-in-determining-if-cardiac-enzymes-play-a-role-in-surveillance-of-possible-hydroxychloroquine-induced-cardiotoxicity/