Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose:
In 2016, the American Academy of Ophthalmology published revised guidelines on HCQ dosing, recommending a maximum daily dose of 5mg/kg actual body weight as higher doses increase the risk of irreversible retinal toxicity. We conducted a QI initiative to increase provider adherence to these guidelines. We aimed to increase dosing compliance by 20 percentage points.
Methods:
HCQ guideline compliance was measured every other week over 10 months by retrospectively reviewing all encounters with non-pregnant patients prescribed HCQ in an adult rheumatology clinic. We recorded date of visit, sex, weight, average daily HCQ dose, any change in dose, and HCQ indication. We used a strict threshold of 5mg/kg in assessing dosing compliance. We also surveyed providers regarding baseline perceptions towards HCQ dosing in clinical practice. Using Plan-Do-Study-Act methodology, we conducted these interventions: grand rounds lectures, modifications of electronic medical record templates, and an email reminder to providers. Charts were analyzed at baseline, between and following interventions.
Results:
In the baseline survey (N=16) when asked, “How worried are you that discussing the new guidelines might lead to non-adherence?” 71% reported little or no concern while 29% of providers were “worried.” Queried whether changing the HCQ dose per guidelines has directly led to an adverse clinical outcome, 23% answered at least one occurrence; 15% recalled having at least one patient suffer significant visual impairment from HCQ.
We analyzed 1218 encounters (87% female) where HCQ was prescribed. The average daily dose was 350mg with 400mg daily being most commonly prescribed (65%). The average weight was 81.5kg. The top three indications for HCQ use were SLE (39%), RA (27%), and UCTD (16%). Baseline guideline compliance was 63% (N=169). During interventions, weekly compliance increased to a peak of 87%, with an overall average of 72% (Figure). Compliance was 99% in patients ≥80kg and 44% in patients <80kg. Dose was decreased in 7% of encounters with a compliance of 62% after reduction. HCQ was started in 10% of encounters with 76% compliance. Dose was increased in 3% of encounters with resultant compliance of 59%. Compliance during interventions was lower among SLE (67%) than other diagnoses (75%).
Conclusion:
Our QI initiative increased compliance by 10 percentage points, short of our aim of 20. This result is not surprising as 29% of providers were worried about flares with dose reduction. A dose decrease did not always result in improved adherence. New initiation of HCQ demonstrated improved adherence compared to the baseline. No one specific intervention appeared most effective. Patients with SLE and weight <80kg were less often within dosing guidelines potentially reflecting a focus for future targeted interventions. Additional studies are also needed to determine flare risk with dose reductions.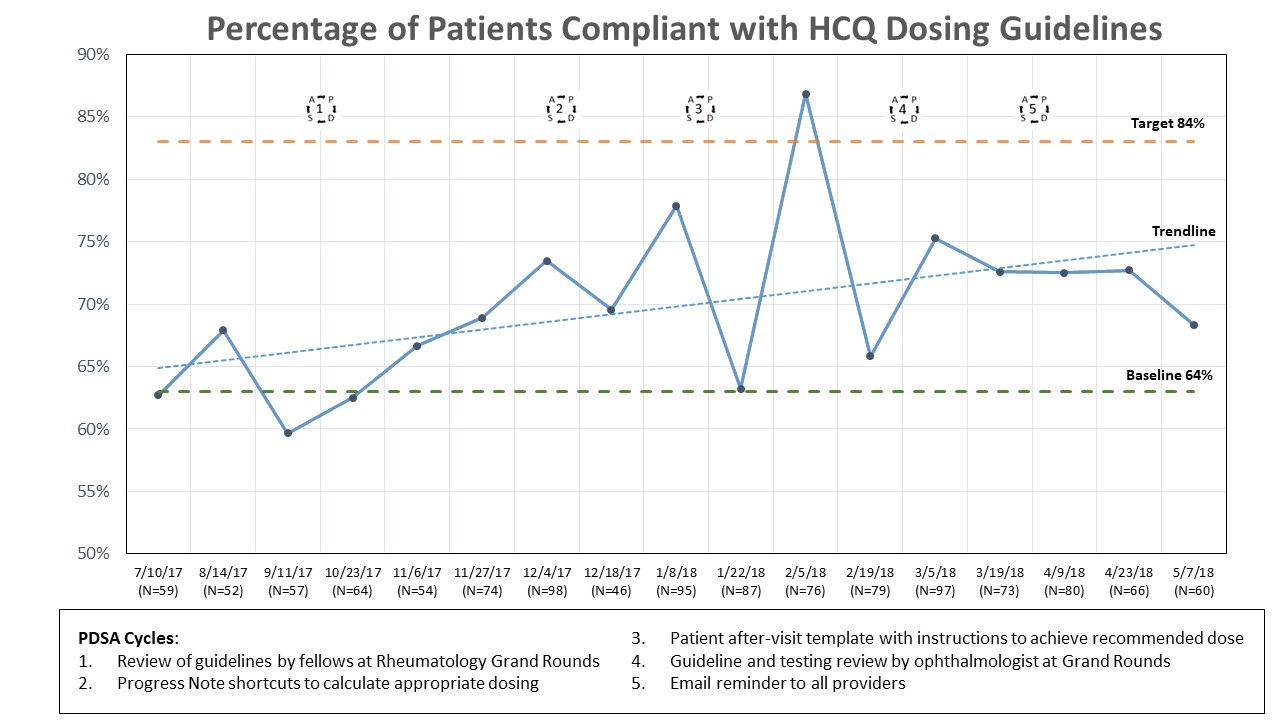
To cite this abstract in AMA style:
Jessee R, Giattino SL, Kapila A, Kaufman K, Golenbiewski J, Andonian BJ, Leverenz D, Criscione-Schreiber L. A Quality Improvement Initiative to Increase Adherence to Hydroxychloroquine Dosing Guidelines at an Academic Medical Center [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/a-quality-improvement-initiative-to-increase-adherence-to-hydroxychloroquine-dosing-guidelines-at-an-academic-medical-center/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-quality-improvement-initiative-to-increase-adherence-to-hydroxychloroquine-dosing-guidelines-at-an-academic-medical-center/
