Session Information
Date: Wednesday, November 8, 2017
Title: Systemic Lupus Erythematosus – Clinical Aspects and Treatment V: Longterm Outcomes
Session Type: ACR Concurrent Abstract Session
Session Time: 9:00AM-10:30AM
Background/Purpose: Two Phase 3, randomized controlled trials (BLISS 52/76) studied the efficacy and safety of belimumab plus standard of care (SoC) in systemic lupus erythematosus (SLE) over 52/76 weeks. A pooled analysis (201223) of the BLISS long-term extension (LTE) studies (BEL112233/NCT00724867; BEL112234/NCT00712933) reported low levels of organ damage accrual in patients who received belimumab plus SoC for 5 years (measured by the Systemic Lupus International Collaborating Clinics [SLICC]/American College of Rheumatology Damage Index [SDI]).1 These studies were open-label with no SoC arm. In order to compare belimumab plus SoC to SoC alone, we conducted a post hoc, propensity score-matched (PSM) analysis (BEL206347) of changes in SDI scores of US patients treated with belimumab plus SoC in the BEL112233 LTE versus patients treated with SoC alone in the Toronto Lupus Cohort (TLC) over 5 years.
Methods: The TLC was identified as the most suitable source of SoC comparator data for the LTE studies.2 A literature review identified potential patient and disease characteristics that impact organ damage for use in the PSM. Propensity scores for each LTE and TLC patient were compared to find an acceptable match (1:1 match, 20% caliper). The primary endpoint was the difference in change in SDI from baseline to Year 5 between patients treated with belimumab plus SoC versus SoC alone, based on the US BLISS LTE and the TLC. A regression augmented inverse PS weighting (IPSW) model tested the robustness of the results. Secondary analyses included parametric hazard models of the time to first increase in SDI score and a proportions test of the magnitude of year-to-year increases in SDI.
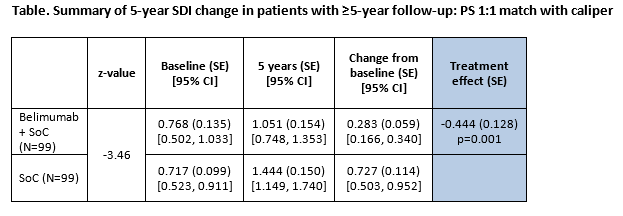
Results: 17 clinical variables were used to calculate the PS; all were well balanced in the sample after PSM. The primary outcome results (5-year SDI change) demonstrated that belimumab was associated with a 0.444 smaller increase (95% confidence interval [CI]: -0.697, -0.191) compared with SoC (Table). The regression augmented IPSW model demonstrated a similar treatment effect estimate (coefficient, -0.450; standard error (SE) 1.115; p>0.001).
Belimumab was associated with a significantly slower rate of organ damage progression (any increase) compared with SoC (hazard ratio: 0.312; SE: 0.087; 95% CI: [0.226, 0.605], p<0.001). Among patients with SDI increases, those treated with SoC alone were more likely to experience an increase of 2+ compared to belimumab plus SoC (30.56% vs 6.06%; p=0.006).
Conclusion: This longitudinal PSM study comparing belimumab plus SoC to SoC alone indicates that belimumab had a clinically significant impact in slowing the rate of organ damage, measured by SDI.
1Bruce IN et al. Lupus 2016;25:699–709; 2Urowitz M et al. Ann Rheum Dis 2017;76(Suppl 2):599
Study funded/conducted by GSK. Editorial assistance provided by Emma Hargreaves, of Fishawack Indicia Ltd, funded by GSK.
To cite this abstract in AMA style:
Urowitz M, Ohsfeldt RL, Wielage R, Kelton KA, Asukai Y, Ramachandran S. A Propensity Score-Matched Study of Organ Damage in Patients with Systemic Lupus Erythematosus from the BLISS Long-Term Extension Trials Versus the Toronto Lupus Cohort: A Post Hoc Longitudinal Analysis [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/a-propensity-score-matched-study-of-organ-damage-in-patients-with-systemic-lupus-erythematosus-from-the-bliss-long-term-extension-trials-versus-the-toronto-lupus-cohort-a-post-hoc-longitudinal-analys/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-propensity-score-matched-study-of-organ-damage-in-patients-with-systemic-lupus-erythematosus-from-the-bliss-long-term-extension-trials-versus-the-toronto-lupus-cohort-a-post-hoc-longitudinal-analys/