Session Information
Date: Friday, November 6, 2020
Title: Vasculitis – Non-ANCA-Associated & Related Disorders II: GCA Clinical & Epidemiology (0514–0518)
Session Type: Abstract Session
Session Time: 4:00PM-4:50PM
Background/Purpose: Two randomized controlled trials [Villiger et al. Lancet 2016; Stone et al. NEJM 2017] demonstrated a glucocorticoid (GC)-sparing effect of tocilizumab (TCZ) of at least 50%. The GUSTO (GCA treatment with Ultra-Short GC and TCZ) trial was set up to unravel the efficacy and safety of TCZ-monotherapy after ultra-short GC treatment in new-onset GCA. Data up to week 24 are presented.
Methods: In this investigator-initiated, single-arm, single-center, open-label clinical trial with Simon’s two stage design, 18 patients with newly diagnosed GCA were enrolled (NCT03745586). Patients received 500mg methylprednisolone intravenously for three consecutive days. Thereafter, GC treatment was discontinued and TCZ (8mg/kg body-weight) was administered intravenously, followed by weekly subcutaneous TCZ injections (162mg) from day 10 until week 52. The primary endpoints included the proportion of patients (i) achieving remission within 31 days and (ii) without relapse at week 24. Remission was defined as disappearance of GCA symptoms; partial remission included the presence of mild symptoms (defined as non-ischemic with NRS < 5/10, reported as mild, not occurring on most days of the week). An interim analysis of the primary endpoint was performed after the first 12 patients reached the primary endpoint, analysis of the 24-week outcome after all patients reached 24 weeks.
Results: Baseline characteristics include 12/18 female patients with a median age of 72 (range 64-78) years, 11/18 with median 1 (range 1-7) day of prior GC-treatment, 15/18 with cranial symptoms (10/18 with jaw claudication, 6/18 with visual involvement), 10/18 with PMR-symptoms, 16/18 with positive cranial ultrasound, 14/18 with aortitis on MRI, 14/18 with vasculitis on cranial MRI and 13/18 with GCA-findings in temporal biopsy.
At the interim analysis, 3/12 patients achieved remission at 31 days and stayed relapse-free until week 24 (25%, 95% confidence interval (CI) 5-57%), i.e. less than necessary to continue to the second stage. We could therefore not reject the null hypothesis that the proportion of responders is smaller than 40% (p=0.92). 11/12 patients achieved remission within 24 weeks after a mean of 74 days (95% CI 50-98) and 10 stayed relapse-free up to 24 weeks (83%, 95% CI 52-98%).
Of the 18 patients recruited up to the time point of the interim analysis, 14 achieved remission within 24 weeks after a mean of 78 days (95% CI 58-97) and 13 stayed relapse-free up to 24 weeks (72%, 95% CI 47-90%).
3/18 patients (17%) were non-responders and started on rescue GC-treatment (2/3 with persistent cranial symptoms including one new-onset anterior ischemic optic neuropathy (AION); 1/3 with persistent PMR symptoms), 2/18 (11%) discontinued the study due to an adverse event (hepatopathy and diverticulitis, respectively; 1/2 after induction of remission). Figure 1 demonstrates remission status over time.
Conclusion: After a 3-days pulse of methylprednisolone, ensuing TCZ monotherapy induced and maintained remission until week 24 in 13/18 patients. Although the primary end-point was not met, the data add an important piece of evidence regarding the potency of blocking the IL-6 pathway in GCA.
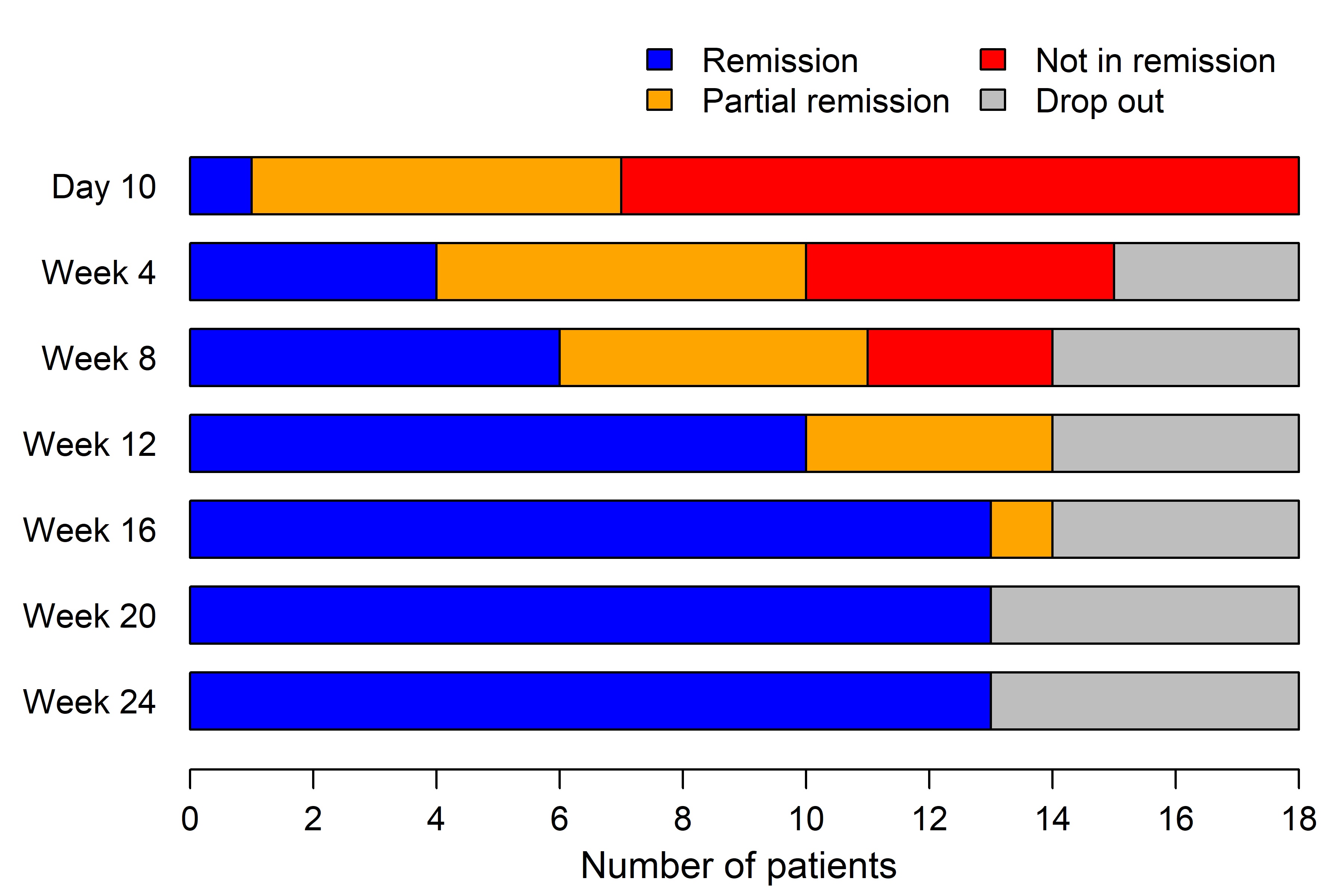 Figure 1: Disease status of patients at each visit (Day 0 – week 24, n=18).
Figure 1: Disease status of patients at each visit (Day 0 – week 24, n=18).
To cite this abstract in AMA style:
Christ L, Seitz L, Buetikofer L, Scholz G, Sarbu A, Amsler J, Kollert F, Reichenbach S, Villiger P. A Proof of Concept Study to Assess the Efficacy of Tocilizumab in Combination with Ultra-Short Glucocorticoid Administration to Treat Newly Diagnosed Giant Cell Arteritis – a 24 Week Analysis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/a-proof-of-concept-study-to-assess-the-efficacy-of-tocilizumab-in-combination-with-ultra-short-glucocorticoid-administration-to-treat-newly-diagnosed-giant-cell-arteritis-a-24-week-analysis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-proof-of-concept-study-to-assess-the-efficacy-of-tocilizumab-in-combination-with-ultra-short-glucocorticoid-administration-to-treat-newly-diagnosed-giant-cell-arteritis-a-24-week-analysis/
