Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: In the setting of today’s heroin epidemic, hepatitis B infection remains a significant public health concern, especially for patients with immunocompromising conditions. With reports of up to 25% mortality associated with hepatitis B reactivation while on immunosuppressive therapy, these patients should be thoroughly screened with hepatitis B surface antibodies (anti-HBsAb) for evidence of immunity, in addition to hepatitis B core antibodies (anti-HBcAb) and hepatitis B surface antigen (HBsAg) for evidence of acute or chronic infection. Our aim was to develop a process to reliably complete hepatitis B screenings on patients receiving intravenously infused biologic medications within the rheumatology division at Cincinnati Children’s Hospital Medical Center (CCHMC).
Methods: Providers within the rheumatology division recognized common barriers to obtaining hepatitis B serology. Eligible patients included all rheumatology patients receiving intravenous biologic therapy between January 2016 and June 2016. Interventions implemented during the study included education of clinic providers and nurses, pre-visit planning resulting in ordering of serology, and the development of physician “talking points” for patients.
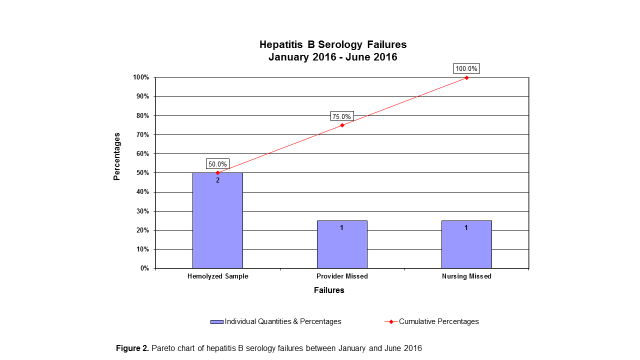
Results: Prior to the intervention, only 4 patients had a complete set of hepatitis B serology obtained within the past year. Anti-HBsAb, anti-HBcAb, and HBsAg serologies were pre-ordered to be drawn at the time of infusion encounters, so there were very few misses (see Figures 1 and 2). The intervention began in January 2016 by targeting patients receiving infliximab, and expanded in April 2016 to include all rheumatology patients receiving infusions of abatacept, belimumab, golimumab, rituximab, and tocilizumab. By June 2016, a total of 109 patients had updated hepatitis B serology. We were able to identify that 71% of patients had negative or indeterminate results for anti-HBsAb and will require repeat vaccination. Much to our surprise, we identified 1 patient on infliximab with a positive anti-HBcAb, presumably from transplacental transmission. This patient is now being monitored by hepatology for re-activation of chronic hepatitis B infection.
Conclusion: We were able to successfully develop a method to update hepatitis B serology for at-risk rheumatology patients on biologic therapy. Next steps will be to develop a process to reliably provide vaccines for patients identified as seronegative; expand this process to screen all patients identified as immunocompromised within rheumatology; and then expand this process to other divisions at CCHMC.
To cite this abstract in AMA style:
Smitherman E, Favier LA, Furnier A, Kramer S, Speer B, Kues J, Danziger-Isakov L, Brady R, Huggins JL. A Process to Obtain Hepatitis B Serology Screening on Immunocompromised Pediatric Rheumatology Patients [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/a-process-to-obtain-hepatitis-b-serology-screening-on-immunocompromised-pediatric-rheumatology-patients/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-process-to-obtain-hepatitis-b-serology-screening-on-immunocompromised-pediatric-rheumatology-patients/