Session Information
Date: Sunday, November 10, 2019
Title: Measures Of Healthcare Quality Poster I: Testing, Screening, & Treating
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Although generally well tolerated, the long-term use of hydroxychloroquine (HCQ) may lead to irreversible and potentially vision-threatening retinal toxicity. The American Academy of Ophthalmology (AAO) issued guidelines in 2011, and again in 2016 that recommended dosing HCQ based on an individual’s body weight, and also outlined how and when to screen for retinal toxicity. While providers have been aware of the potential side effects of HCQ for decades, studies have shown that many patients continue to receive higher than recommended doses. We conducted a pragmatic randomized trial to assess the utility of a new e-prescribing (eRX) interface to support appropriate dosing of HCQ.
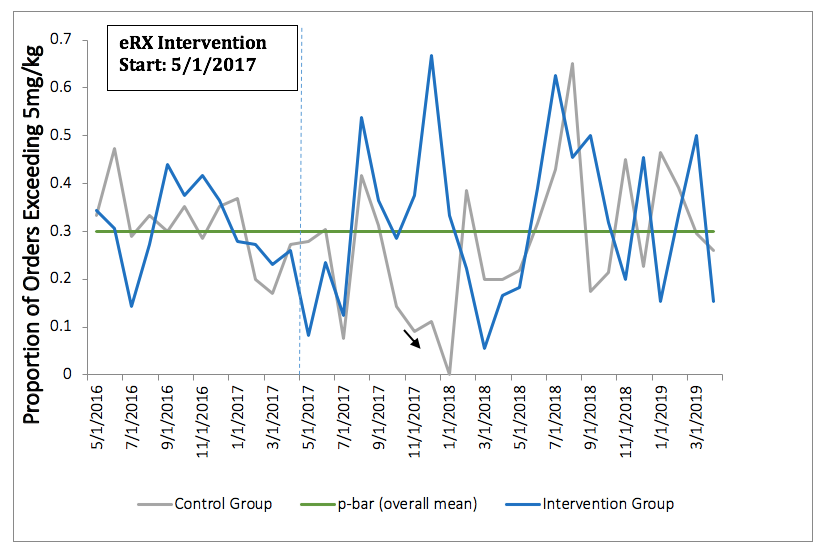
Methods: Rheumatology providers at a large university health center were randomized into either an intervention (n=12) or control (n=12) group. In the intervention group, providers were presented with an eRX interface which automatically pulled in a patient’s weight from the electronic health record each time a new order for HCQ was entered. A suggested HCQ dosage was provided in accordance with the 2016 AAO recommendation, defined as < 5.0 mg/kg (Figure 1). The interface rounded dosage to the nearest half tablet. The control group received no change to the standard ordering interface. Chi-squared tests were used to compare the proportion of all HCQ prescription dosages > 5.0 mg/kg prior to (May 1, 2016-April 30, 2017) and all new orders after eRX implementation (May 1, 2017-April 31, 2019). Mixed effects models were used to examine the association between group assignment and HCQ dosage > 5.0mg/kg, controlling for patient factors and clustering by provider.
Results: A total of 551 patients contributed 1,664 HCQ prescription orders. Most patients were white (62%) and female (87%), with a mean age of 43.2 (+/- 18.5). Prior to the eRX intervention, 69% of HCQ orders were within guidelines across groups. We found no significant difference between groups with respect to the proportion of prescribed dosages > 5.0mg/kg both before and after the eRX implementation (p=0.71 and p=0.45, respectively) (Table 1). After introduction of the eRX interface, the intervention group prescribed 32% of HCQ prescriptions at higher than recommended doses, similar to the control group (29%). The null association remained after controlling for patient level factors and clustering by provider. Our control chart also did not show any significant change in prescribing patterns over time (Figure 1).
Conclusion: This study demonstrates that the eRX interface in its current state was insufficient to significantly reduce HCQ dosing to levels recommended in current guidelines. However, the small sample size, rounding of dosage to nearest half tablet, and restriction to new HCQ orders may have limited our ability to detect differences between groups. Additional iterations of the eRX interface should address these issues. Qualitative studies could also help elucidate reasons for lack of improvement, including clinical reasons such as uncertainty about guideline cutpoints or concerns about efficacy, or implementation issues such as the design of the eRX interface and associated workflows.
To cite this abstract in AMA style:
Gianfrancesco M, Murray S, Evans M, Schmajuk G, Yazdany J. A Pragmatic Randomized Trial to Improve Safe Dosing of Hydroxychloroquine [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/a-pragmatic-randomized-trial-to-improve-safe-dosing-of-hydroxychloroquine/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-pragmatic-randomized-trial-to-improve-safe-dosing-of-hydroxychloroquine/