Session Information
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
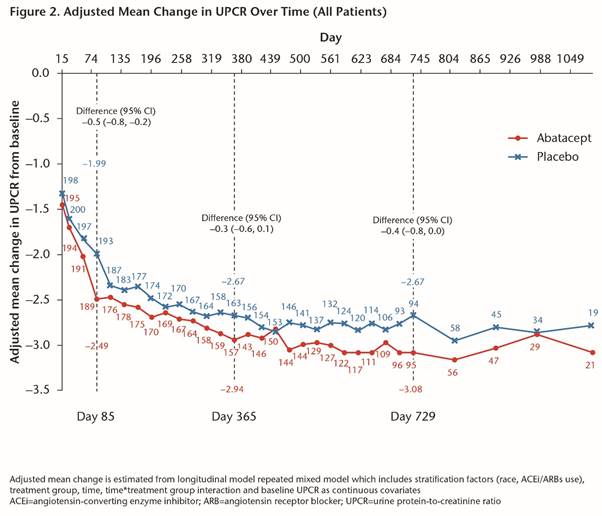
Methods: This was a 24-month (M), randomized, Phase III, multicenter, double-blind study with a blinded long-term extension. Patients (pts) were randomized 1:1 to pbo or IV ABA 30 mg/kg for 3M, followed by ABA ~10 mg/kg every 4 wks on a background of mycophenolate + corticosteroids (CS). Primary endpoint, complete renal response (CR) at 1 yr, was a composite measure requiring maintenance of glomerular filtration rate (GFR), urine protein-to-creatinine ratio (UPCR) ≤0.5, absence of urinary cellular casts and CS ≤10 mg/day. We report all blinded data up to 3 yrs of tx (as-observed analysis).
Conclusion: The study failed to meet its primary endpoint of higher CR rate in pts with active LN after 1 yr of abatacept tx. Abatacept-treated pts had more rapid improvement in proteinuria, which led to more sustained CR up to 3 yrs. There were favorable efficacy and safety profiles in Yr 2 and 3 of abatacept tx.
To cite this abstract in AMA style:
Dooley MA, Appel GB, Furie R, Wofsy D, Takeuchi T, Malvar A, Doria A, Romero-Díaz J, Chan TM, Elegbe A, Jayne D, Maldonado MA. A Phase III Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Abatacept or Placebo on Standard of Care in Patients with Active Class III or IV Lupus Nephritis [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/a-phase-iii-randomized-double-blind-placebo-controlled-study-to-evaluate-the-efficacy-and-safety-of-abatacept-or-placebo-on-standard-of-care-in-patients-with-active-class-iii-or-iv-lupus-nephritis/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-phase-iii-randomized-double-blind-placebo-controlled-study-to-evaluate-the-efficacy-and-safety-of-abatacept-or-placebo-on-standard-of-care-in-patients-with-active-class-iii-or-iv-lupus-nephritis/