Session Information
Date: Tuesday, November 14, 2023
Title: (1913–1944) Miscellaneous Rheumatic & Inflammatory Diseases Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: JS005 is a novel anti-IL-17A monoclonal antibody with unique patented complementarity-determining region (CDR) sequences. This Phase Ib/II study (NCT05344248) aimed to evaluate the safety, tolerability, efficacy and pharmacokinetic characteristics of JS005 in patients with moderate to severe plaque psoriasis.
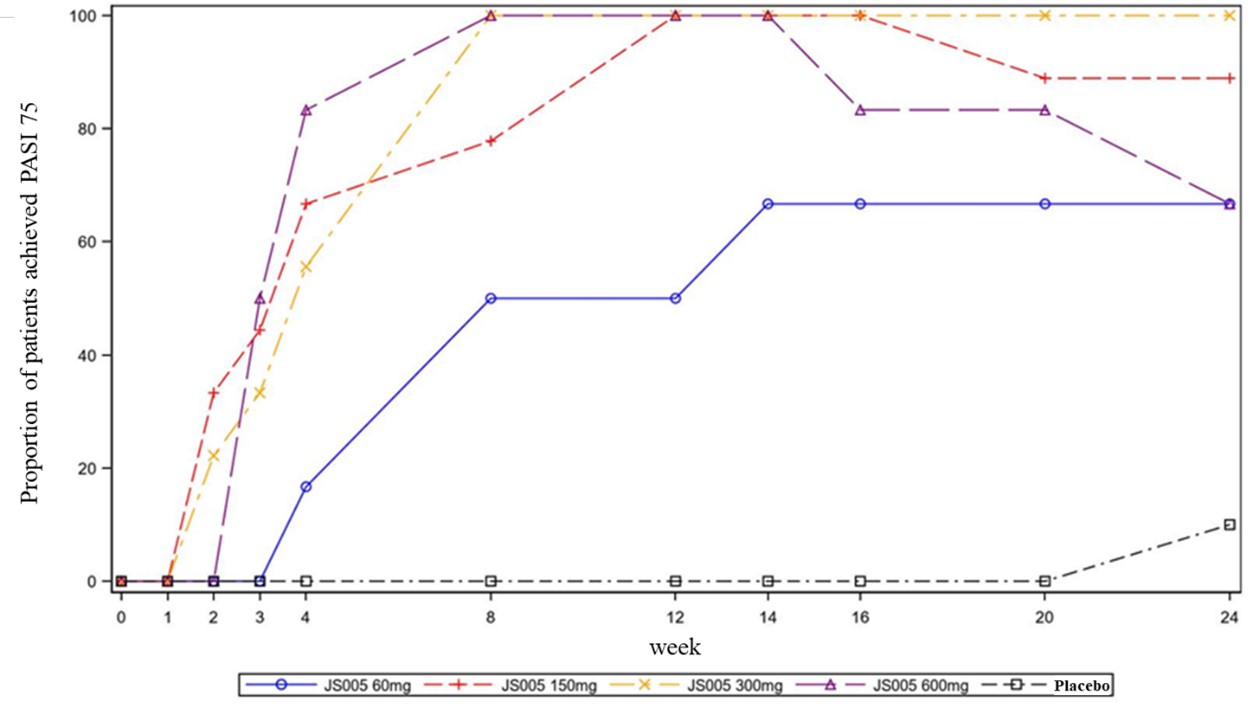
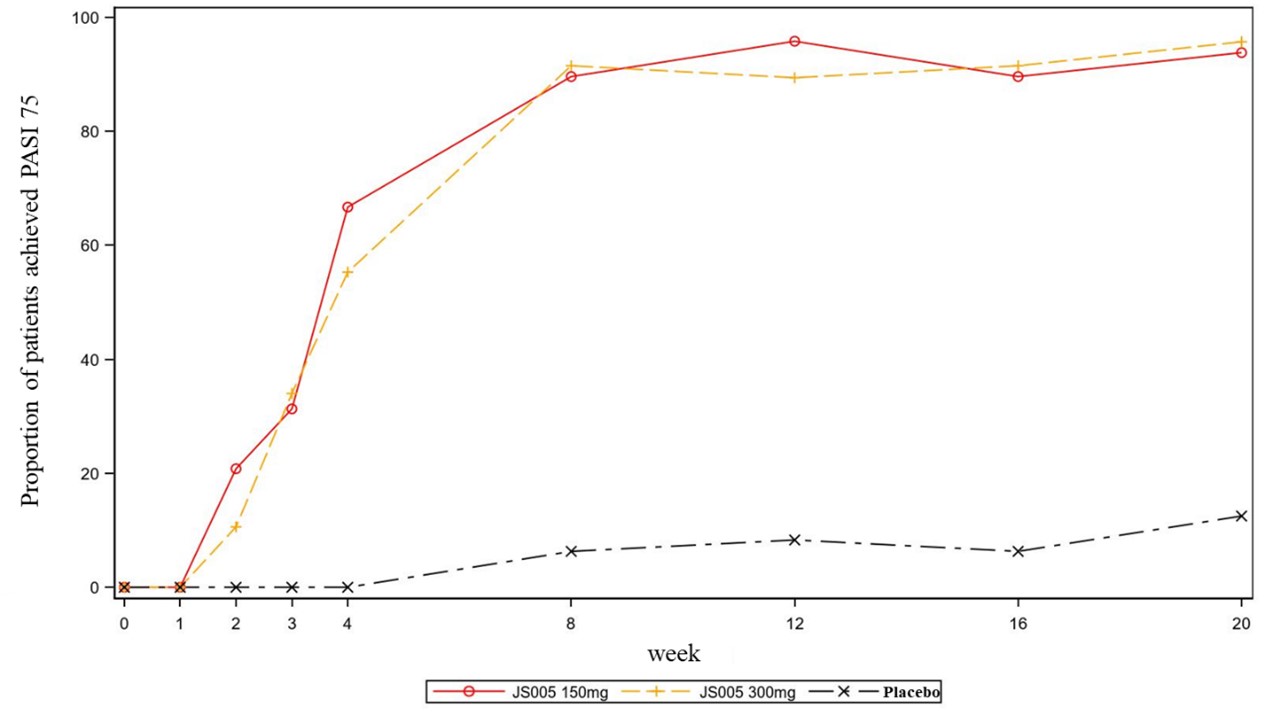
Methods: This study was composed of a dose-escalation phase Ib trial and a multicenter, double-blind, placebo-controlled phase II trial. Moderate to severe plaque psoriasis patients were enrolled to receive subcutaneous JS005 60mg (n=6), 150mg (n=9), 300mg (n=9), 600mg (n=6) and placebo (n=10) in the phase Ib trial. In the phase II trial, moderate to severe plaque psoriasis patients aged 18 to 75 years were randomized in a 1:1:1 ratio to receive subcutaneous JS005 at 150mg (n=48), 300mg (n=47) or placebo (n=48) once a week (QW) from Week 0 to Week 4, and once every 4 weeks (Q4W) from Week 5 to Week 12. Follow-up period was continued for 8 weeks after the last dose. The primary endpoint was the proportion of patients with at least 75% improvement in Psoriasis Area and Severity Index (PASI 75) from baseline at Week 12.
Results: A total of 40 and 143 patients were enrolled in this phase Ib and phase II trials, respectively. In the phase Ib trial, JS005 was well tolerated in moderate to severe plaque psoriasis patients, the incidence of Treatment Emergent Adverse Events (TEAEs) was similar across groups (p >0.05). Results from full analysis set (FAS) showed significantly higher response rates of PASI 75 with JS005 60mg (50%, p< 0.0001), 150mg (100%, p< 0.0001), 300mg (100%, p< 0.0001) and 600mg (100%, p< 0.0001) than with placebo (0%) at Week 12 (Fig. 1). In the phase II trial, the proportions of patients achieved PASI 75 at week 12 were significantly higher in the JS005 150mg group (95.8%, p< 0.0001) and 300mg group (89.4%, p< 0.0001) than in the placebo group (8.3%) (Fig. 2). The incidence of TEAEs was 62.5%, 63.8%, and 64.6% in the JS005 150mg, 300mg and placebo groups, respectively. The severity of TEAEs in JS005 150mg and 300mg groups were all CTCAE grade 1 and Grade 2. The most common drug related TEAEs in JS005 group were hyperuricemia (6.3%), blood uric acid increase (5.3%), and hypertriglyceridemia (5.3%). There were no AEs that led to study withdrawal and permanent treatment discontinuation, and no serious AE or death events occurred.
Conclusion: This phase Ib/II study demonstrated that JS005 was highly effective and well tolerated in moderate to severe plaque psoriasis.
To cite this abstract in AMA style:
Cai L, Mu Z, Tao X, Zhang L, Zhang C, Li Y, Zhang G, Zhang F, Dong X, Li C, Chen A, Wu Z, Zhu Y, Zhang M, Liu J, Zhang J. A Phase Ib/II Randomized, Double-blind, Placebo-controlled Study of Novel anti-IL-17A Monoclonal Antibody JS005 in Patients with Moderate to Severe Psoriasis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/a-phase-ib-ii-randomized-double-blind-placebo-controlled-study-of-novel-anti-il-17a-monoclonal-antibody-js005-in-patients-with-moderate-to-severe-psoriasis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-phase-ib-ii-randomized-double-blind-placebo-controlled-study-of-novel-anti-il-17a-monoclonal-antibody-js005-in-patients-with-moderate-to-severe-psoriasis/